Nội dung toàn văn Decision 3355/QD-BYT 2021 plan for launching of COVID19 vaccination campaign for 2021 2022
MINISTRY OF HEALTH | SOCIALIST REPUBLIC OF VIETNAM |
No. 3355/QD-BYT | Hanoi, July 08, 2021 |
DECISION
PROMULGATING PLAN FOR LAUNCHING OF COVID-19 VACCINATION CAMPAIGN FOR 2021-2022
MINISTER OF HEALTH
Pursuant to the Government’s Decree No. 75/2017/ND-CP dated 20/6/2017 on functions, duties, powers and organizational structure of Ministry of Health;
Pursuant to the Government’s Resolution No. 21/NQ-CP dated 26/2/2021 on purchase and use of COVID-19 vaccines;
Pursuant to Decision No. 3043/QD-BYT dated 24/6/2021 by the Ministry of Health on establishment of Steering Committee for Nationwide Launching of COVID-19 Vaccination Campaign;
At the request of General Director of General Department of Preventive Medicine, Ministry of Health,
HEREBY DECIDES:
Article 1. Promulgated together with this Decision is the plan for launching of COVID-19 vaccination campaign for 2021-2022 (hereinafter referred to as “Plan”).
Article 2. The Plan shall provide the basis for formulation and launch of COVID-19 vaccination plans of units and local governments. The plan will be revised as appropriate to the pandemic’s situation and vaccine provision capacity.
Article 3. This Decision takes effect from the date on which it is signed and supersedes Decision No. 1467/QD-BYT dated 05/3/2021 by the Ministry of Health approving 2021 - 2022 COVID-19 vaccination plan and Decision No. 1464/QD-BYT dated 05/3/2021 by the Ministry of Health on guidelines on receiving, preserving, distributing and using COVID-19 vaccines.
Article 4. Heads of Office of the Ministry of Health, General Department of Preventive Medicine, Drug Administration of Vietnam, Medical Services Administration, Department of Planning and Finance, Department of Communication and Emulation, Commendation, Institutes of Hygiene and Epidemiology, Pasteur Institutes, National Institute for Control of Vaccines and Biologicals, Departments of Health and relevant units shall implement this Decision./.
| MINISTER |
PLAN
LAUNCHING OF COVID-19 VACCINATION CAMPAIGN FOR 2021-2022
(Enclosed with Decision No. 3355/QD-BYT dated 08/7/2021 by Minister of Health)
COVID-19 vaccination is a crucial solution for the fight against COVID-19 and socio-economic development. Other countries around the world have launched the largest vaccination program in history[1].
In Vietnam, the Ministry of Health has approached vaccine sources via different channels such as directly discussing with vaccine manufacturers, international organizations, embassies in Vietnam and Vietnam’s ambassadors in other countries. So far, 105 million doses from different sources have been secured for Vietnam[2].
Vietnam strives to achieve herd immunity by the end of 2021 or the start of 2022 with approximately 70% of Vietnam’s population vaccinated. To reach this target and as a large amount of vaccines will arrive in Vietnam soon, we need to launch the largest national vaccination campaign in history with the participation of healthcare, military and police forces and ministries. This vaccination campaign must take place concurrently in vaccination facilities nationwide, including public and private facilities, units affiliated and unaffiliated with the healthcare sector, etc.
For launching of the largest vaccination campaign in history involving all forces and citizens and using vaccines provided by multiple sources in large quantity and with different storage conditions, the Ministry of Health has formulated a plan for launching of the COVID-19 vaccination campaign on the basis of Vietnam’s situation and estimated vaccine provision capacity.
I. BASES FOR PLAN FORMULATION
- Law on Prevention and Control of Infectious Diseases No. 03/2007/QH12 dated 21/11/2007.
- Law on Pharmacy No. 105/2016/QH13 dated 06/4/2016.
- Directions of the Politburo and Secretariat in Official Dispatch No. 50-CV/VPTW dated 19/02/2021 by Office of the Central Committee.
- Conclusion No. 07-KL/TW dated 11/6/2021 by the Politburo on some key tasks in COVID-19 prevention and control and socio - economic development.
- The Government’s Decree No. 104/2016/ND-CP dated 01/7/2016 on vaccination.
- The Government’s Resolution No. 21/NQ-CP dated 26/2/2021 on purchase and use of COVID-19 vaccines.
- The Government’s Resolution No. 58/NQ-CP dated 08/6/2021 on Government’s regular meeting of May 2021.
- Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health elaborating some Articles of the Government’s Decree No. 104/2016/ND-CP dated 01/7/2016 on vaccination.
- Directions of the Government and Prime Minister[3].
II. OBJECTIVES
1. General objective: proactively prevent and control COVID-19 by administering COVID-19 vaccines to at-risk persons and communities.
2. Specific objectives:
- At least 50% of persons aged 18 or older receive COVID-19 vaccines in 2021.
- More than 70% of the population receive COVID-19 vaccines by the end of the first quarter of 2022.
- Ensure safe COVID-19 vaccination.
III. RULES, TIME, RECIPIENTS AND SCOPE
1. Rules
- Launch the campaign in all communes across the country.
- Use all qualified vaccines from different sources at the same time to increase vaccination coverage.
- Use vaccines before expiry date to prevent wastefulness.
- Mobilize the political system to join the vaccination campaign; mobilize all forces, including facilities affiliated and unaffiliated with the healthcare sector, police forces, military forces, socio-political organizations, ministries and mass organizations (Youth Union, Women’s Union, etc.), to support vaccination.
- Ensure high vaccination coverage among people of COVID-19 vaccination age (more than 90%).
- Ensure absolute safety in vaccination.
2. Time: from July 2021 to April 2022.
3. Vaccine recipients:
All people of vaccination age recommended by vaccine manufacturers, with priority given to front-line participants in COVID-19 containment and economic development:
a) Workers of public and private healthcare facilities and the healthcare sector;
b) Participants in COVID-19 prevention and control (members of steering committees for COVID-19 prevention and control at all levels, workers in quarantine areas, persons involved in contact tracing and epidemiological investigation, community COVID teams, volunteers, reporters, etc.);
c) Military forces;
d) Police forces;
dd) Vietnamese diplomatic officials and staff seconded overseas and families thereof; workers in diplomatic missions, consular posts and international organizations operating in Vietnam[4];
e) Customs staff and officials involved in entry and exit operations;
g) Providers of essential services such as aviation, transport, tourism; power supply and water supply;
h) Teachers, workers and students of educational and training institutions; young doctors; workers in administrative units and agencies; lawyer organizations, notary organizations, bidding organizations, etc. frequently meeting many people;
i) Persons having chronic illness and persons aged 65 and above;
k) Persons living in infected areas;
l) Poor people and social policy beneficiaries;
m) Persons seconded to work or study abroad by competent authority or wishing to work or study abroad; foreign experts working in Vietnam;
n) Workers of enterprises[5] (including enterprises in industrial parks and export-processing zones, transport businesses, credit institutions, tourism businesses, etc.), providers of essential services such as lodgings, food and beverages, banking services, healthcare, pharmacies, medical equipment providers, etc., retail and wholesale establishments, markets and construction works and families thereof, and people living in tourism areas;
o) Dignitaries and sub-dignitaries;
p) Freelance workers;
q) Other recipients decided by the Minister of Health or Chairperson of provincial People’s Committee and proposed by vaccine sponsors of the Ministry of Health;
Establishments, organizations and enterprises of the abovementioned vaccine recipients comprise both public and private entities.
4. Scope: nationwide, with priority given to:
- Infected provinces and cities; in infected provinces and cities, prioritize those in infected areas.
- Provinces and cities which are parts of key economic regions or in which economic development schemes of the Government are piloted.
- Provinces and cities having multiple industrial parks and/or industry clusters and large number of workers and residents.
- Provinces and cities having borders, heavy traffic and/or international border checkpoints.
5. Method for organization
Launch the vaccination campaign in eligible vaccination facilities at all levels (fixed and mobile vaccination points).
IV. CONTENT
1. Vaccine provision
- Obtain qualified vaccines from different sources such as assistance from the COVAX Facility and other countries; assistance from organizations and individuals and purchase from different manufacturers.
- The Ministry of Health will distribute vaccines to provinces and cities according to vaccine provision schedule following the order of priority mentioned in Section 4 of Part III, which will be published on the information portal of the vaccination campaign.
1.1. Setting up cold chain systems
- Complete cold chain systems of units affiliated to the Ministry of National Defense and improve capacity of cold chain systems of the Expanded Program on Immunization.
- Formulate training documents and organize training in vaccine receipt, transport and storage.
- Ensure that the whole cold chain systems meet GSP requirements.
1.2. Receiving vaccines and vaccination materials
National Institute of Hygiene and Epidemiology shall complete procedures for import of COVID-19 vaccines and vaccination materials and equipment from other countries and sponsors or receipt of these items from distributors in Vietnam according to Decision No. 1345/QD-BYT dated 19/02/2021 by the Ministry of Health on purchase, receipt, storage and distribution of COVID-19 vaccines and vaccination materials and Decision No. 2566/QD-BYT dated 26/5/2021 by the Ministry of Health on purchase of COVID-19 vaccination materials and equipment and receipt of sponsored COVID-19 vaccination materials and equipment.
1.3. Transporting and storing vaccines and vaccination materials
Each vaccine batch shall be delivered to vaccination points within 03 days after the batch release certificate is issued.
1.3.1. Vaccines requiring storage from 2° to 8°C
These vaccines shall be stored at 2° to 8°C throughout the receipt, storage and transport processes at all levels (according to the diagrams in Appendix 1 enclosed therewith).
a) From March to July 2021
- Employ existing cold chain systems of the Expanded Program on Immunization to transport and store vaccines. Vaccines must be stored at 2° to 8°C throughout the receipt, storage and transport processes at all levels.
- Units with sufficient cold chain systems shall store vaccines using their systems on vaccination days. For units without sufficient cold chain systems, the provincial CDC/ district-level medical center shall provide vaccines for each vaccination session or lend cold boxes and vaccine carriers to them. Vaccines unused at the end of each vaccination period shall be returned to the provincial CDC/ district-level medical center.
- The Ministry of National Defense shall take charge and cooperate with the Expanded Program on Immunization in transporting vaccines.
b) From August 2021 onwards
- Employ cold chain systems of units affiliated to the Ministry of National Defense for vaccine storage and transport.
- Within 02 days after issuance of a vaccine distribution decision by the Ministry of Health, units of the Ministry of National Defense shall receive vaccines from the national warehouse and cooperate with the Department of Health in dispensing the vaccines to district-level medical centers or vaccination points according to local plans.
- Units with sufficient cold chain systems shall store vaccines using their systems on vaccination days. For units without sufficient cold chain systems, the provincial CDC/ district-level medical center shall provide or lend cold boxes and vaccine carriers to them. Vaccines unused at the end of each vaccination period shall be temporarily stored at the provincial CDC/ district-level medical center and notified to units of the Ministry of National Defense for coordination.
1.3.2. Vaccines requiring storage at low/ultra-low temperature and possible to store at 2° to 8°C
a) From March to July 2021
- Employ available cold chain systems of the Expanded Program on Immunization to transport and store vaccines (according to the diagram in Appendix 2 enclosed therewith).
- The provider shall transport vaccines at low/ultra-low temperature and hand over the vaccines to the provincial CDC for storage at 2°C to 8°C.
- The provincial CDC shall cooperate with the provincial Military Command (where necessary) in providing vaccines for district-level medical centers within 01 day after receiving the vaccines.
- Units with sufficient cold chain systems for storage at 2°C to 8°C shall store vaccines using their systems on vaccination days. For units without sufficient cold chain systems, the provincial CDC shall provide vaccines for each vaccination session or provide or lend cold boxes and vaccine carriers to them.
- Vaccines unused at the end of each vaccination period shall be returned to the district-level medical center or provincial CDC so that they can be provided for other units in the province. These units will use the vaccines during the period in which they may be stored at 2° to 8°C where necessary.
- The Ministry of National Defense shall take charge and cooperate with the Expanded Program on Immunization in transporting vaccines.
b) From August 2021 onwards
- Employ cold chain systems of units of the Ministry of National Defense to transport and store vaccines (according to the diagram in Appendix 2 enclosed therewith).
- The provider shall transport vaccines at low/ultra-low temperature and hand over the vaccines to units of the Ministry of National Defense for storage at 2°C to 8°C.
- Within 02 days after issuance of a vaccine distribution decision by the Ministry of Health, units of the Ministry of National Defense shall receive vaccines from the provider and cooperate with the Department of Health in dispensing the vaccines to district-level medical centers according to local plans.
- The district-level medical center shall dispense the vaccines to vaccination points in the district according to local plans.
- Vaccines unused at the end of each vaccination period shall be temporarily stored at the provincial CDC/ district-level medical center and notified to units of the Ministry of National Defense for coordination. Vaccines shall be stored at 2°C to 8°C for the period of time recommended by the manufacturer.
Note: vaccines that have been stored at 2°C to 8°C must not be stored at low temperature.
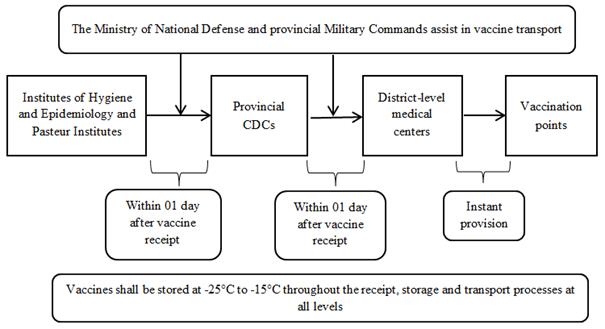
1.3.3. Vaccines requiring storage from -25° to -15°C
These vaccines shall be stored at -25° to -15°C throughout the receipt, storage and transport processes at all levels (according to the diagrams in Appendix 3 enclosed therewith).
a) From March to July 2021
- The vaccine provider shall deliver vaccines to Institutes of Hygiene and Epidemiology/ Pasteur Institutes. Institutes of Hygiene and Epidemiology/ Pasteur Institutes shall receive and hand these vaccines over to provincial CDCs, which will cooperate with provincial Military Commands (where necessary) or mobilize district-level medical centers to transport and dispense the vaccines to vaccination points immediately according to local plans.
- Units of the Ministry of National Defense shall take charge and cooperate with the Expanded Program on Immunization in transporting vaccines.
b) From August 2021 onwards
The provider shall transport vaccines at low temperature and hand over the vaccines to units of the Ministry of National Defense for storage at -25°C to -15°C. These units shall cooperate with Departments of Health in dispensing the vaccines to district-level medical centers, which will dispense the vaccines to vaccination points using cold boxes and dry ice according to local plans.
Note: leftover defrosted vaccines must be disposed of as per regulations.
2. Vaccination organization
2.1. Improving capacity of vaccination systems
- Review, invest in and prepare facilities, equipment, materials, cold chains, workforce, etc. for fixed and mobile vaccination facilities.
- Draw up lists of eligible vaccination facilities, including public and private facilities and facilities affiliated and unaffiliated with the healthcare sector; provide plans for mobilizing all local private vaccination facilities to cooperate in vaccination where necessary.
- Develop documents, programs and plans; units shall cooperate in providing healthcare workers with training in vaccine storage, transport and use and adverse event monitoring for each type of vaccine.
2.2. Organizing vaccination sessions
- Use existing systems of the Expanded Program on Immunization, vaccination facilities of ministries, public and private vaccination service providers and other facilities eligible for vaccination.
- In case it is necessary to accelerate vaccination, arrange clusters of mobile vaccination points in factories and industrial parks to administer vaccines to multiple recipients at the same time.
- Vaccination facilities shall administer vaccines at different times within the day, at different tables and with multiple vaccination points to ensure safe distancing; utilize information technology in vaccination and assign officials to support application of information technology to vaccination.
- Treatment facilities shall administer vaccines to recipients who require special monitoring according to guidelines of the Ministry of Health.
3. Ensuring safety in vaccination
- Formulate training documents and organize training in providing instructions on screening, handling of vaccine injury and vaccination safety.
- Screen proactively to identify recipients to be vaccinated in treatment facilities.
- Central hospitals, provincial hospitals and district-level hospitals and medical centers shall establish their own emergency aid teams and at least 01 mobile emergency aid team for every 3-4 vaccination points, especially in hard-to-access communes. During COVID-19 vaccination periods, provincial hospitals must dedicate some ICU beds (at least 5 beds/hospital) for handling of vaccine injury.
- Other vaccination facilities (commune-level medical stations, hospitals, infirmaries, healthcare establishments, etc. affiliated to ministries, vaccination service providers, etc.) must have equipment for immediate emergency aid and plans for assisting emergency aid if necessary.
- The 5K requirements, distancing measures and measures against COVID-19 must be imposed at vaccination points.
4. Applying information technology to vaccination management
To publish information in a transparent manner and enable individuals, organizations, units and local governments to launch the COVID-19 vaccination campaign, units and local governments shall use the platform for COVID-19 vaccination management to launch the campaign. This platform consists of 04 components: (1) vaccination information portal at https://tiemchungcovid19. gov. vn; (2) vaccination support system; (3) management support system; and (4) electronic health book application. To be specific:
4.1. Vaccine recipient management
- Information on vaccination registration, vaccination plans, vaccination schedules and relevant mass announcements shall be updated on the vaccination information portal continuously.
- Vaccination registration, health declaration and updating on events following immunization shall be carried out via the “electronic health book” application on mobile phones and the information portal.
- In case of limited vaccine distribution, listing of vaccine recipients and vaccination schedule arranging shall be carried out before announcing that vaccination registration is open. In case of sufficient vaccines, vaccine recipient lists may be drawn up after announcing that vaccination registration is open if there are sufficient vaccines for large-scale vaccination.
4.2. Vaccination facility management
- Publish and regularly update information on vaccination locations, vaccination table numbers and in-charge persons on the information portal of the COVID-19 vaccination campaign at https://tiemchungcovid19.gov.vn.
- Vaccination facilities must update information on quantities of vaccine doses received, administered and leftover and these figures shall be updated on the page for management information from steering committees for COVID-19 vaccination campaign on the COVID-19 vaccination management platform.
- The management support system of steering committees for COVID-19 vaccination campaign shall update number of vaccinated persons, number of postponed vaccinations and number of persons granted vaccination certificates (first dose and second dose if any) of each vaccination facility online.
4.3. Management of vaccine receipt, transport and storage
- Submit and update reports of steering committees for COVID-19 vaccination campaign on number of distributed vaccine doses and information that provides the basis for distribution to localities. Consolidate reports of local governments on plans for dose distribution for each distribution phase of steering committees for COVID-19 vaccination campaign.
- The management support system shall update information on quantity and time of receipt, dispatch and re-receipt of vaccine doses by batch number assigned by the manufacturer of general warehouses and relevant warehouses in receipt, storage and transport systems designated by steering committees for COVID-19 vaccination campaign online.
- Units and healthcare facilities involved in receipt, transport and storage shall update information, quantities and reports on the COVID-19 vaccination management platform.
4.4. Vaccination session management
Information related to steps to be taken shall be directly updated to the vaccination support system of the COVID-19 vaccination management platform according to the 04 following steps: Reception/Screening and confirmation of vaccination eligibility/Vaccine administration and post-vaccination monitoring/Certificate issuance.
5. Communications
5.1. Communications content
- Disseminate guidelines and policies of the Communist Party and the State, directions of the National Assembly and the Government on COVID-19 vaccination, focusing on instructional documents of the Politburo, the Secretariat, the Government and the Prime Minister, especially the Government’s Resolution No. 21/NQ-CP dated 26/02/2021 on purchase and use of COVID-19 vaccines.
- Encourage people to support COVID-19 vaccination in the spirit of “COVID-19 vaccination is a right of the individual and a responsibility to the community”; encourage people to receive vaccines when it is their turns; encourage people to support Vietnam’s COVID-19 vaccine fund.
- Disseminate plans for launching of COVID-19 vaccination campaign from central to local level; benefits of COVID-19 vaccination to COVID-19 containment and recommendations for safe COVID-19 vaccination as well as monitoring and handling of events following vaccination.
- Commend individuals exemplary in implementing vaccination plans.
5.2. Communications activities
- Employ mass communication to promptly provide accurate information on the vaccination campaign, encourage people to receive vaccines when it is their turns, provide recommendations for safe COVID-19 vaccination as well as monitoring of events following vaccination and encourage people to support the vaccination campaign and Vietnam’s COVID-19 vaccine fund via articles, reports, talk shows, online discussions, television and radio programs, etc.
- Cooperate with press organizations and ministries in promoting COVID-19 vaccination campaign in a vigorous, consistent and efficient manner.
- Formulate and upload messages, recommendations and communicating documents on COVID-19 vaccination to the electronic data warehouse for communicating documents on COVID-19 prevention, control and vaccinations, which will be used by local governments to encourage people to get vaccinated.
- Launch communications campaigns concerning COVID-19 vaccination on social network sites; promote the vaccination campaign on social network sites and via online media.
- Set up hotlines of the Ministry of Health and local governments to promptly provide information and advice on COVID-19 vaccination for people.
- Promote exemplary cases in COVID-19 vaccination operations.
- Organize training in communications about COVID-19 vaccination and adverse events following COVID-19 vaccination for press agencies, healthcare workers and forces participating in the vaccination campaign.
6. Managing syringes and biomedical waste after vaccination
- After every vaccination session, handle syringes and biomedical waste according to regulations in Joint Circular No. 58/2015/TTLT-BYT-BTNM dated 31/12/2015 by the Ministry of Health and Ministry of Natural Resources and Environment stipulating regulations on biomedical waste management and Document No. 102/MT-YT dated 04/03/2021 on guidelines for management of biomedical waste in COVID-19 vaccination.
- Vaccination facilities shall provide plans for collecting and handling syringes and biomedical waste at vaccination points according to regulations of the Ministry of Health. Used vaccine vials must be disposed of, recorded and reported.
7. Controlling vaccine and vaccination quality
7.1. Vaccination monitoring
- Carry out inspection and monitoring before, during and after the vaccination campaign.
- National steering committee for vaccination, Ministry of Health, Departments of Health and relevant units shall monitor and supervise COVID-19 vaccination operations.
- Expedite and give directions to ensure that vaccination operations are on schedule.
7.2. Vaccine quality control
- Control vaccine quality before use.
- Control vaccine quality during use and collect samples for quality inspection on a periodic or ad hoc basis.
7.3. Reporting on vaccination results
- Report on vaccination results on a daily basis and, at the end of the vaccination campaign, report on vaccine receipt and use and vaccination results.
- Use the health records application of the Ministry of Health for reporting.
8. Funding
8.1. Central government budget
- Vaccines and some vaccination materials such as vaccine administering syringes, vaccine mixing syringes and safety boxes.
- Improvement of central-level cold chain systems.
- Cooperation with local governments in transporting vaccines to provincial warehouses or vaccination points.
- Training for provincial level.
- Development of communication messages and launching of communications activities at central level.
- Compensation for some deceased cases according to the Government’s Decree No. 104/2016/ND-CP dated 01/7/2016.
8.2. Local government budget
- Costs of transporting vaccines from provincial warehouses to vaccination points (in case the Ministry of Health only transports vaccines to provincial warehouses); vaccine storage equipment according to regulations.
- Costs of organizing the vaccination campaign, including allowances for vaccination staff, costs of consumables (besides those covered by the Ministry of Health), costs of electricity, water, fuels, biomedical waste treatment, environmental hygiene directly supporting vaccination, etc.
- Training for district-level medical centers and local vaccination points.
- Local communications activities.
- Vaccine purchase (for vaccines purchased by local governments).
8.3. Funding sources
- State budget (including central government budget and local government budget, with central government budget providing support for local government budget according to regulations in Clause 1 Article 3 of the Government’s Resolution No. 21/NQ-CP dated 26/02/2021);
- COVID-19 vaccine fund;
- Sponsorship, aid and contributions from Vietnamese and foreign organizations and individuals and other lawful funding sources for central and local levels.
V. IMPLEMENTATION
1. Central level
Establish a steering committee for nationwide launching of COVID-19 vaccination campaign. This committee shall have the Minister of Health as the head and leaders of the Ministry of Health, Ministry of National Defense, Ministry of Public Security, Ministry of Transport, Ministry of Information and Communications and Central Committee of Ho Chi Minh Communist Youth Union as the deputy heads. This committee shall comprise subcommittees for vaccine receipt, transport and storage; vaccine administration; vaccination safety; vaccine quality control; application of information technology to COVID-19 vaccination management and communications.
1.1. Subcommittee for vaccine receipt, transport and storage shall:
- Direct units affiliated to the Ministry of Health and Ministry of National Defense to cooperate in completing cold chain systems of the Ministry of National Defense so that they can be used starting from August 2021.
- Direct the Expanded Program on Immunization and units of the Ministry of National Defense to organize receipt of COVID-19 vaccines at points of receipt upon import (airports, etc.) and transport of COVID-19 vaccines from points of receipt upon import (airports, etc.) to storage locations managed by the Ministry of National Defense or Ministry of Health or locations mobilized where necessary.
- Direct transport of COVID-19 vaccines from storage locations managed by units of the Ministry of National Defense or Ministry of Health to vaccination locations throughout the country according to regulations on vaccine management.
- Direct training and supervise vaccine storage and transport at all levels to ensure compliance with schedule and regulations.
1.2. Subcommittee for vaccine administration shall:
- Cooperate with the subcommittee for vaccine receipt, transport and storage in directing handover and receipt of vaccines at vaccination points throughout the country; take charge of distributing vaccine doses between units, provinces and cities.
- Direct launching of the COVID-19 vaccination campaign at mobile vaccination points nationwide.
- Cooperate with the subcommittee for application of information technology to COVID-19 vaccination management in directing aggregation of daily vaccination data to promptly direct acceleration of vaccine administration.
- Mobilize the Youth Union and other forces to support the vaccination campaign.
- Direct formulation of training documents and training in organization of vaccination sessions and monitoring of adverse events following immunization.
1.3. Subcommittee for vaccination safety shall:
- Cooperate with the subcommittee for vaccine administration in directing supervision and inspection of vaccination safety at vaccination points throughout the country.
- Take charge and cooperate with regulatory bodies in formulating guidelines on vaccine administration and handling of adverse events following vaccination.
- Direct emergency aid for adverse events following COVID-19 vaccination.
- Analyze and evaluate treatment of adverse events following COVID-19 vaccination.
- Direct imposition of the 5K requirements, distancing measures and measures against COVID-19 at vaccination points.
- Direct formulation of training documents and training in vaccination safety.
1.4. Subcommittee for vaccine quality control shall:
- Direct vaccine quality supervision and assurance of vaccine quality from receipt at airports to transport, storage and distribution to vaccination points.
- Direct formulation of training documents and training in vaccine quality control.
1.5. Subcommittee for application of information technology to COVID-19 vaccination management shall:
- Direct development of an information technology application for management of the whole vaccination campaign, including vaccine receipt, transport, storage and distribution, vaccine recipient management and vaccination coverage.
- Direct application of information technology and use of personal health records for vaccination; and development of a system for vaccination certification.
- Cooperate with the subcommittee for vaccine administration in directing aggregation of data on COVID-19 vaccine demand and quantity and vaccination progress of vaccination points and publishing the data on the digital map of the vaccination campaign.
- Direct communications activities for the national COVID-19 vaccination campaign.
- Direct formulation of training documents, organize training and supervise task performance.
2. Local level
2.1. Provincial People’s Committees shall:
- Establish steering committees for COVID-19 vaccination campaigns at all levels. These committees shall be headed by leaders of People's Committees at all levels and consist of the same subcommittees as the steering committee for nationwide launching of COVID-19 vaccination campaign, which may be changed according to local situation.
- Approve plans for launching of COVID-19 vaccination campaign in their provinces to ensure efficiency and safety and prevent wastefulness.
- Invest in cold chain materials and equipment supporting COVID-19 vaccination.
- Use all distributed vaccines; do not waste any vaccine.
- Direct military units, police forces, healthcare forces, transport forces, etc. to cooperate in launching the vaccination campaign.
2.2. Departments of Health shall:
- Advise on and propose plans for launching COVID-19 vaccination campaign to provincial People's Committees; invest in improving local vaccination systems, organize the campaign using all distributed vaccines and ensure vaccination safety.
- Take charge and cooperate with district-level People’s Committees and relevant units in drawing up lists of eligible vaccination facilities and formulating plans for mobilizing local vaccination facilities for the campaign.
- Take charge and cooperate with units in distributing vaccines to vaccination points.
- Take charge and cooperate with units of the Ministry of National Defense and relevant units in distributing vaccines within their provinces.
APPENDIX 1:
TRANSPORT OF VACCINES REQUIRING STORAGE FROM 2° TO 8°C
1. From March to July 2021
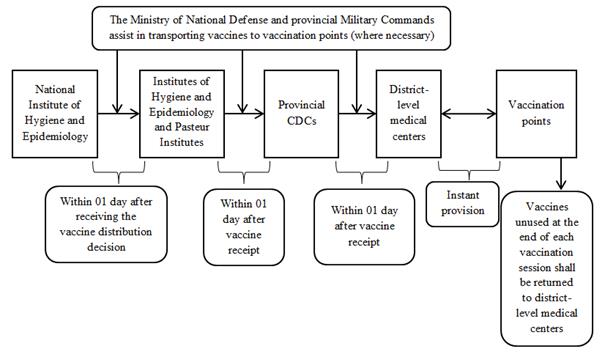
2. From August 2021 onwards
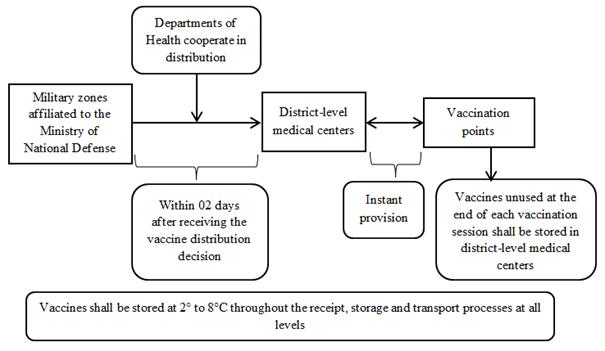
APPENDIX 2:
TRANSPORT OF VACCINES REQUIRING STORAGE AT LOW/ULTRA-LOW TEMPERATURE AND POSSIBLE TO STORE AT 2° TO 8°C (PFIZER, MODERNA AND JANSSEN VACCINES)
1. From March to July 2021
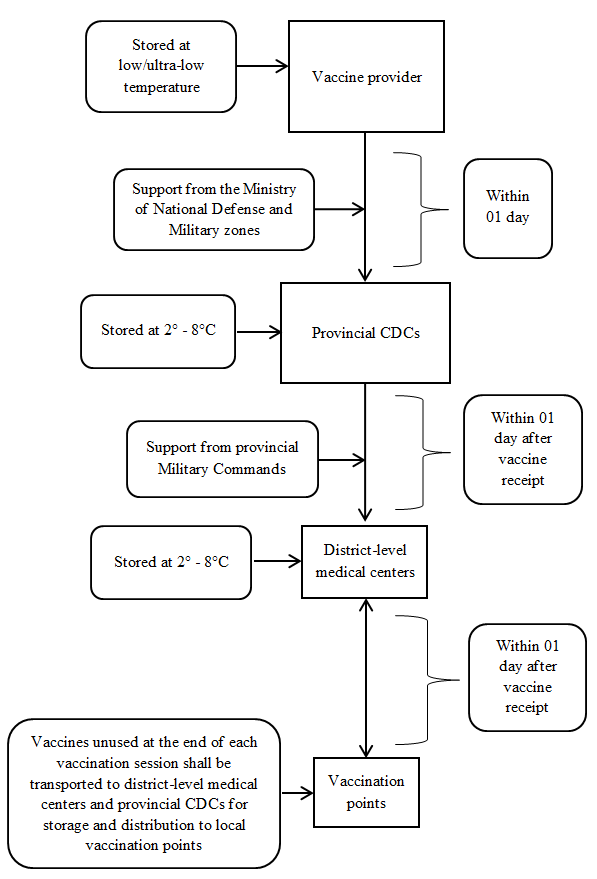
2. From August 2021 onwards
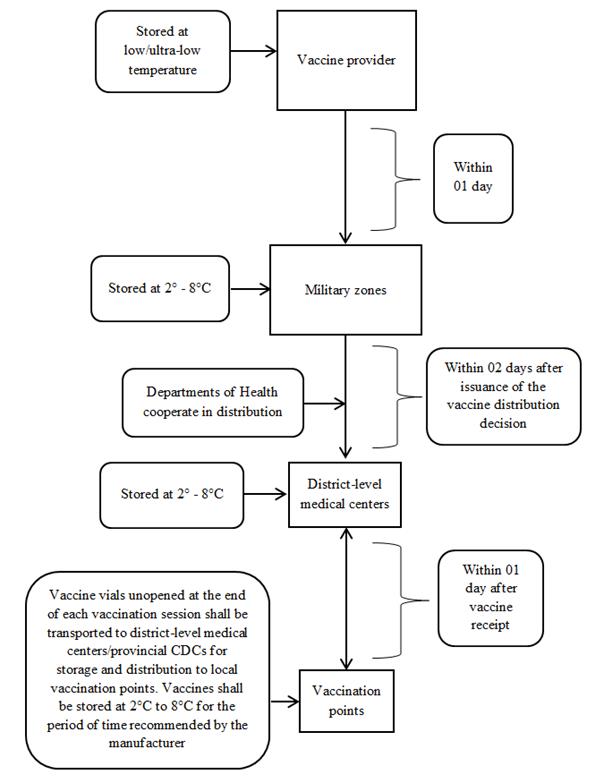
APPENDIX 3:
TRANSPORT OF VACCINES REQUIRING STORAGE FROM -25° TO -15°C (SPUTNIK V VACCINE IN FROZEN FORM)
1. From March to July 2021
2. From August 2021 onwards
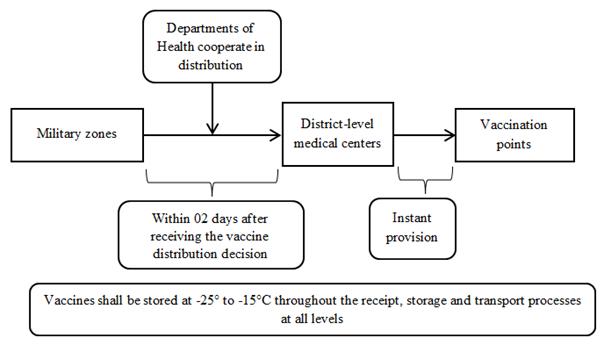
APPENDIX 4:
LIST OF VACCINE STORAGES IN MILITARY ZONES AND PROVINCES/CITIES
No. | Military zone | Province/City |
1 | Storage 01: Capital High Command | Hanoi |
2 | Storage 02: Military zone 1 (6 provinces) | Cao Bang, Lang Son, Bac Kan, Thai Nguyen, Bac Ninh and Bac Giang |
3 | Storage 03: Military zone 2 (9 provinces) | Vinh Phuc, Phu Tho, Dien Bien, Lai Chau, Tuyen Quang, Ha Giang, Yen Bai, Lao Cai and Son La |
4 | Storage 04: Military zone 3 (8 provinces and 1 city) | Hai Phong, Quang Ninh, Thai Binh, Nam Dinh, Ha Nam, Ninh Binh, Hoa Binh, Hai Duong and Hung Yen |
5 | Storage 05: Military zone 4 (6 provinces) | Nghe An, Ha Tinh, Quang Binh, Quang Tri, Thua Thien Hue and Thanh Hoa |
6 | Storage 06: Military zone 5 (10 provinces and 1 city) | Da Nang, Quang Nam, Quang Ngai, Binh Dinh, Phu Yen, Khanh Hoa, Ninh Thuan, Kon Tum, Gia Lai, Dak Lak and Dak Nong |
7 | Storage 07: Military zone 7 (9 provinces and 1 city) | Ho Chi Minh City, Binh Duong, Dong Nai, Long An, Tay Ninh, Binh Phuoc, Lam Dong, Binh Thuan and Ba Ria Vung Tau |
8 | Storage 08: Military zone 9 (11 provinces and 1 city) | Can Tho, Hau Giang, An Giang, Kien Giang, Tien Giang, Ben Tre, Dong Thap, Vinh Long, Tra Vinh, Soc Trang, Bac Lieu and Ca Mau |
[1] As of 25/6/2021, 2,84 billion COVID-19 vaccine doses have been administered in 214 countries and territories; 805.58 million people have been fully vaccinated. Common vaccines are AZ (730 million doses), Pfizer (590 million doses), Sinovac (630 million doses), Sinopharm (430 million doses), Moderna (210 million doses), etc.
[2] 38,9 million doses are provided by the COVAX Facility and 5 million doses are provided by other countries (Australia, China and Japan); agreements to purchase 31 million doses of Pfizer/BioNTech vaccine and 30 million doses of AZ vaccine from VNVC have been signed.
[3] - Notification No. 164/TB-VPCP dated 31/12/2020 by the Office of the Government on the Prime Minister’s conclusions in meeting of standing Government members on COVID-19 vaccines.
- Notification No. 47/TB-VPCP dated 17/3/2021 by the Office of the Government on the Prime Minister’s conclusions in meeting of standing Government members on adding persons eligible for free COVID-19 vaccination.
- Notification No. 57/TB-VPCP dated 23/3/2021 by the Office of the Government on the Prime Minister’s conclusions in meeting of standing Government members on COVID-19 prevention and control.
- Notification No. 89/TB-VPCP dated 01/5/2021 by the Office of the Government on the Prime Minister’s conclusions in meeting of standing Government members on COVID-19 prevention and control.
- Notification No. 137/TB-VPCP dated 30/5/2020 by the Office of the Government on the Prime Minister’s conclusions in online national meeting on COVID-19 prevention and control.
[4] Notification No. 47/TB-VPCP dated 17/3/2021 by the Office of the Government on the Prime Minister’s conclusions in meeting of standing Government members on adding persons eligible for free COVID-19 vaccination.
[5] Notification No. 137/TB-VPCP dated 30/5/2020 by the Office of the Government on the Prime Minister’s conclusions in online national meeting on COVID-19 prevention and control.