Nội dung toàn văn Circular No. 21/2014/TT-BKHCN measurement of pre-packed goods
|
MINISTRY OF SCIENCE
AND TECHNOLOGY |
SOCIALIST REPUBLIC
OF VIETNAM |
|
No. 21/2014/TT-BKHCN |
Hanoi, July 15, 2014 |
CIRCULAR
MEASUREMENT OF PRE-PACKED GOODS
Pursuant to the Law on Measurement dated November 11, 2011;
Pursuant to the Government's Decree No. 86/2012/NĐ-CP dated October 19, 2012 on guidelines for the Law on Measurement;
Pursuant to the Government's Decree No. 89/2006/NĐ-CP dated August 30, 2006 of goods labels;
Pursuant to the Government's Decree No. 20/2013/NĐ-CP dated February 26 2013 defining the functions, tasks, entitlements and organizational structure of the Ministry of Science and Technology;
At the request of Director of the Directorate for Standards, Metrology, and Quality,
The Minister of Science and Technology promulgates a Circular on measurement of pre-packed goods.
Chapter I
GENERAL PROVISIONS
Article 1. Scope
1. This Circular specifies the measurements of pre-packed goods, including: technical requirements applied to measurements of pre-packed goods; list of pre-packed goods Group 2; true quantity markings; announcement of true quantity markings on labels of pre-packed goods Group 1; certificates of eligibility to use true quantity markings on labels of pre-packed goods Group 2.
2. This Circular does not apply to pre-packed goods being medicines, goods given customs privileges or exemptions; temporarily imported goods; goods in transit; goods in bonded warehouses; goods processed by Vietnamese companies for foreign traders; imported components of machinery and equipment serving project of investment; classified goods; goods serving emergency needs; goods serving special measurement activities for national defense and security.
Article 2. Regulated entities
1. Entities that manufacture, import, transport, sell pre-packed goods.
2. Measurement authorities and relevant entities.
Article 3. Interpretation of terms
In this Circular, the terms below are construed as follows:
1. Pre-packed goods are goods of which the quantity is expressed as a unit of weight, volume, area, length, or pcs, and are packed and quantified without the presence of the buyers.
2. Pre-packed goods Group 1 are pre-packed goods that are not on the list in Clause 3 of this Article.
3. Pre-packed goods Group 2 are pre-packed goods that are sold in large quantities or have high value that are likely to cause disputes over measurements among parties, or considerably affect human health or the environment. The list of pre-packed goods Group 2 are provided in Article 6 of this Circular.
4. A unit or pack of pre-packed goods means a collection of package and quantity of goods contained therein.
5. Package is the material used to wrap pre-packed goods, unless it is part of the goods in nature.
6. Real quantity (Qr) means the quantity of pre-packed goods in the package and determined with proper instruments.
7. Unconformable unit of pre-packed goods means a unit of pre-packed goods of with the real quantity smaller than the minimum quantity (Qmin).
8. Minimum quantity (Qmin) is the difference between nominal quantity (Qn) and tolerance (T).
Qmin = Qn - T
Tolerance is specified in Appendix IV enclosed herewith.
9. Nominal quantity (Qn) means the quantity of pre-packed goods written on the goods label.
10. Average value (Xtb) of a quantity of pre-packed goods means the value prescribed in Clause 3 Article 3 of the Government's Decree No. 86/2012/NĐ-CP dated October 19, 2012 on guidelines for the Law on Measurement.
11. Batch of pre-packed goods (hereinafter referred to as batch) means a collection of identical units of pre-packed goods that are manufactured, imported, transported, or sold with a nominal quantity under similar conditions from which samples are taken for assessment of conformity with technical measurement requirements.
12. Batch size (N) means the total number of units of pre-packed goods in the batch and is determined as follows:
a) Where goods are manufactured, the batch sized is expressed as the number of units of pre-packed goods manufactured in an hour;
b) Where goods are imported, the batch size is expressed as number of units of pre-packed goods imported;
c) Where goods are transported or sold, the batch size is expressed as the number of units of pre-packed goods on the means of transport, in the storage or store;
d) A batch size must not exceed (100,000).
13. Sample size (N) means the number of units of pre-packed goods taken as samples from in the batch for assessment.
14. Number of unconformable units of pre-packed goods means the number of units of pre-packed goods that are not conformable with requirements, which is determined during assessment of the batch of pre-packed goods.
15. Solvent means the liquid or gas that is wrapped together with pre-packed goods and is discarded when pre-packed goods are used.
16. Dry contents means the true quantity of pre-packed goods in a liquid solvent.
17. Manufacturer of pre-packed goods means the entity that carries out the manufacturing, packaging, or bottling stage in Vietnam.
18. Importer of pre-packed goods means the entity that imports or entrusts the import of pre-packed goods.
19. Other terms are construed as interpreted in Article 3 of the Law on Measurement.
Chapter II
TECHNICAL MEASUREMENT REQUIREMENTS APPLIED TO QUANTITIES OF PRE-PACKED GOODS, LIST OF PRE-PACKED GOODS GROUP 2
Article 4. Technical requirements applied to quantities of pre-packed goods
1. Quantity of pre-packed goods on the label
a) The quantity of pre-packed goods on the label is nominal quantity
b) The nominal quantity (Qn) of pre-packed goods shall be printed or fixed directly on pre-packed goods or their packages or labels;
c) The position of nominal quantity must be noticeable and legible when goods are displayed in normal conditions;
d) If the nominal quantity is “net weight” or “true volume”, the world “approximate” or “minimum” must not be used e.g. approximate net weight or minimum true volume;
dd) The nominal quantity shall be expressed as a value together with a legal unit of measurement or pcs; there must be a space between the number and the unit;
e) The nominal quantity of pre-packed goods measured a unit of weight or volume as follows:
- Pre-packed goods that is solids, liquefied gas, frozen, aerosols, or contained together with compressed air shall be measured in a unit of weight.
- Goods that are in liquid form shall be measured in a unit of volume;
- Goods that are in emulsion form shall be measured in a unit of weight or volume;
g) The unit of nominal quantity depends on the quantity as prescribed in Appendix I enclosed herewith.
h) The minimum height of the text and number expressing the nominal quantity must comply with Appendix II enclosed herewith;
i) When a unit of pre-packed goods contains multiple packs of pre-packed goods and a nominal quantity that can be sold retail, the total nominal quantity of the unit of pre-packed goods, the number of packs and nominal quantity in a pack must be specified.
Example: A box of coffee contains 10 packs; the net weight of each pack is 20 g. The nominal quantity of the box of coffee shall be written as follows:
200 g (10 packs x 20 g)
i) When a unit of pre-packed goods contains multiple packs of pre-packed goods and a nominal quantity that can be sold retail, the total nominal quantity of the unit of pre-packed goods, the number of packs and nominal quantity in a pack must be specified.
Example: A pack of shoe polish contains 02 boxes of black polish that weigh 15 g each ; a box of brown polish that weights 25 g; a box of white polish that weighs 15 g. Nominal quantity of the pack shall be written as follows:
Black polished: 2 boxes x 15 g; brown polish: 1 box x 25 g; white polish: 1 box x 15 g.
Total: 4 boxes (70 g).
l) With regard to pre-packed goods in solvent, the weight of dry contents and total weight shall be written.
2. Requirements applied to shapes, sizes, and other aspects of pre-packed goods packages
a) The shape, size, and structure of packages of pre-packed goods must not cause confusion about the quantity of pre-packed goods contained therein (such as having secondary bottom, partition, secondary cover, etc.);
b) Pre-packed goods must fill the volume of the package, unless there is a difference between the volume of the package and volume of pre-packed goods contained therein because of:
- Protection of pre-packed goods;
- Requirements of the equipment used for wrapping pre-packed goods;
- Requirements of the transport of pre-packed goods;
- The nature of pre-packed goods (aerosols cans, etc.)
c) If the quantity of pre-packed goods is written at various positions on the package, the quantity at each of the positions must comply with regulations in Clause 1 of this Article;
d) Information about an additional or complimentary quantity of pre-packed goods must be written together with the nominal quantity on the label, unless the manufacturers states clearly that such additional or complementary is included in the nominal quantity of pre-packed goods.
Article 5. Requirements applied to quantities of pre-packed goods
1. Mean value Xtb
a) If the sample size (n) equals (=) the batch size (N), mean value (Xtb) of real quantity of the units of pre-packed goods in the batch must satisfy the expression below:
Xtb ³ Qn
b) If the sample size is smaller than the batch size (n < n),="" the="" mean="" value="">tb) must satisfy the expression below:
Xtb ³ Qn - k.s
Where: s is the squared deviation of real quantity calculated using the formula in Appendix III enclosed herewith.
K is a coefficient in Appendix III enclosed herewith.
2. Number of unconformable units of pre-packed goods;
a) In case the sample size is equals (=) the batch size, the following requirements must be satisfied:
- The number of unconformable units of pre-packed goods must not exceed 2.5% of the batch size;
- There is no unconformable unit of pre-packed goods of which the deficit is larger than twice the tolerance prescribed in Appendix IV enclosed herewith.
b) If the sample size is smaller than the batch size, the following requirements must be satisfied:
- The number of unconformable units of pre-packed goods must not exceed the permissible limit in Table 1, Table 2 (if samples are taken where goods are manufactured, imported, transported, or sold), or Table 3 (if packages must be opened to take samples) in Appendix III enclosed herewith.
- There is no unconformable unit of pre-packed goods of which the deficit is larger than twice the tolerance prescribed in Appendix IV enclosed herewith.
Article 6. List of pre-packed goods group 2.
1. The list of pre-packed goods Group 2 contains names of specific pre-packed goods.
2. According to proposals of Ministries and regulatory bodies, the Ministry of Science and Technology shall decide the pre-packed goods Group 2 on the List prescribed in Clause 1 of this Article.
Chapter III
TRUE QUANTITY MARKING
Article 7. True quantity marking
1. True quantity marking is the symbol showing that the quantity of pre-packed goods is conformable with Article 3 and Article 5 of this Circular.
2. The true quantity marking is the letter V, uppercase, bold, using “Times New Roman” font. The height of the letter is ≥ 3mm.
Article 8. Use of true quantity marking
1. Pre-packed goods Group 1 shall bear the true quantity marking if requirements in Article 4 and Article 5 are fulfilled, and the use of the true quantity marking is announced by a facility that meet the requirements in Article 10 or Article 11 as prescribed in Section 2 Chapter IV of this Circular.
2. Pre-packed goods Group 1 shall bear the true quantity marking if requirements in Article 4 and Article 5 are fulfilled, and such fulfillment is announced by a facility that meet the requirements in Article 10 or Article 11 as prescribed in Section 2 Chapter IV of this Circular.
Article 9. Expression of true quantity marking on pre-packed goods
1. The true quantity marking shall be printed or fixed directly on the goods, packages or labels thereof where it is noticeable and legible.
2. The color of the true quantity marking shall be the same as that of the nominal quantity and is placed before the nominal quantity of pre-packed goods. There must be a space between the true quantity marking and the nominal quantity.
e.g. V 800 g or V 500 ml.
Chapter IV
ANNOUNCEMENT OF TRUE QUANTITY MARKING, CERTIFICATION OF ELIGIBILITY FOR TRUE QUANTITY MARKING
Section 1: REQUIREMENTS APPLIED TO TRUE QUANTITY MARKING USERS
Article 10. Requirements applied to manufacturers of pre-packed goods
1. The facility is established in accordance with law.
2. The infrastructure satisfies the requirements below:
a) There are sufficient instruments for quantifying pre-packed goods (applied to manufacturers) or measuring instruments for assessing the conformity of pre-packed goods quantity (applied to facilities that assess the conformity themselves; the measuring instruments are inspected and calibrated periodically as prescribed;
b) The workplace, environmental conditions, and other conditions are conformable with Clause 4 of this Article.
3. There are adequate technicians in charge of quantity control measures as prescribed in Clause 4 of this Article.
4. Measures for quality control of measuring instruments and measuring tasks (hereinafter referred to as quantity control measures) are taken to ensure fulfillment of technical measurement requirements of pre-packed goods.
5. Documents about inspection and calibration results, documents about technical assessment of quantity of pre-packed goods (carried out by the facility itself or the certifying organization hired by the facility as prescribed in Article 16 of this Circular) are retained.
Article 11. Requirements applied to importers of pre-packed goods
1. The true quantity marking is shown on labels of pre-packed goods Group 2.
2. Quantity control measures are taken to ensure fulfillment of technical measurement requirements of quantity of pre-packed goods.
Section 2: Announcement of use of true quantity marking
Article 12. Procedures for announcing the use of true quantity marking
1. Every facility that satisfies the requirements in Article 10 and Article 11 of this Circular shall make 02 copies of the announcement of the use of the true quantity marking on pre-packed goods labels (hereinafter referred to as announcement) using the form No. CBDĐL in Appendix V enclosed herewith, then submit them, whether directly or by post, to the Department of Standards, Metrology and Quality of the province where the headquarter of the facility is located.
2. If the announcement is not satisfactory, the Department of Standards, Metrology and Quality, within 03 working days from the receipt of the announcement, shall notify the applicant of necessary additions or adjustments.
If the announcements are not satisfactory, within 30 working days from their receipt, the Department of Standards, Metrology and Quality shall notify the applicant of necessary additions or adjustments.
3. If the announcements are satisfactory, within 05 working days from their receipt, the Department of Standards, Metrology and Quality shall append its seal onto the announcements and return one of them to the applicant.
4. At the request of Director of the Directorate for Standards, Metrology, and Quality shall monitor and manage the announcements.
Article 13. Retention of announcements
1. Announcement documents include:
a) The announcement bearing the seal of the Department of Standards, Metrology, and Quality;
b) Written regulations on quantity control measures for ensuring fulfillment of technical measurement requirements of pre-packed goods.
c) Documents about inspection and calibration results, documents about technical assessment of quantity of pre-packed goods, and documents about implementation of other quantity control measures;
2. Documents shall be kept for 02 more years after they are replaced, invalidated, or expired.
Article 14. Adjustments to the announcement
1. When there is any change to the contents of the announcement submitted, or the announcement is lost or damaged, the facility shall follow the procedures in Article 12 of this Circular.
2. The announcement shall be numbered continuously from the first submission.
Article 15. Invalidation of the announcement
1. The announcement of the use of true quantity marking on pre-packed goods shall be invalidated in the following cases:
a) The facility commits serious violations prescribed in Article 25 of this Circular;
b) The facility sends a notification of invalidation of the announcement to the local Department for Standards, Metrology and Quality;
c) The facility goes bankrupt or is dissolved as prescribed by law.
2. In the case prescribed in Point b Clause 1 of this Article, the Department of Standards, Metrology, and Quality shall append its signature and seal on the notification, send it back to the facility, and take a note in the logbook.
Section 3. CERTIFICATION OF ELIGIBILITY TO USE TRUE QUANTITY MARKING
Article 16. Certifying bodies
1. the Directorate for Standards, Metrology, and Quality shall certify the eligibility to use the true quantity marking of facilities that satisfy requirements for manufacturing or importing pre-packed goods nationwide.
2. Provincial Department of Standards, Metrology, and Quality shall certify the eligibility to use the true quantity marking of local facilities that satisfy requirements for manufacturing or importing pre-packed goods.
Article 17. Application for certification
Applicants that satisfy corresponding requirements in Article 10 or Article 11 of this Circular shall compile an application and submit it to the certifying body directly or by post. The application consists of:
1. An application for certification of eligibility to use the true quantity marking on pre-packed goods labels (using the form No. 2 ĐNCN in Appendix V enclose herewith).
2. Copies (certified by the applicant) of the Decision on Establishment or Certificate of Business registration issued by a competent authority.
3. A document compiled by the head of the facility that contains regulations on quantity control measures to ensure fulfillment of technical measurement requirements of quantity of pre-packed goods.
4. Documents about inspection and calibration results, documents about technical assessment of quantity of pre-packed goods, and documents about implementation of other quantity control measures.
Article 18. Application processing
1. If the application is not satisfactory, within 05 working days, the certifying body shall send the applicant a written notification of necessary additions.
If the applicant fails to complete the application within 90 days from the notification date, the certifying body is entitled to reject that application.
2. Within 30 working days from the receipt of the satisfactory application, the certifying body shall issue a decision to establish an inspectorate and carry out an inspection at the facility as prescribed in Article 19 of this Circular (this regulation is applied to manufacturers of pre-packed goods);
3. Within 15 working days from the receipt of the satisfactory application, the certifying body shall issue the certificate of eligibility to use the true quantity marking on labels of pre-packed goods (hereinafter referred to as certificate) to the applicant in accordance with Article 20 of this Circular.
Article 19. Inspection at the facility
1. Inspection shall be carried out at the facility by the experts of the inspectorate.
2. The inspectorate is established by the head of the certifying body. The inspectorate shall report the result to the head of the certifying body.
3. Composition of the inspectorate and responsibilities of members thereof
a) An inspectorate has a chief and members. The inspectorate shall consist of not fewer than 02 people; the chief and members must have certificate of training in assessment of pre-packed goods measurement issued by the Directorate for Standards, Metrology, and Quality;
b) The chief shall organize the operation of the inspectorate, evaluate the management system; give tasks to members; convene and chair meetings of the inspectorate, and approve the inspection result before requesting the head of the certifying body to issue the certificate.
c) Other members shall perform their tasks take responsibility for their performance.
4. The inspection involves evaluation of the fulfillment of requirements in Article 10 or Article 11 of this Circular of the facility.
5. Directorate for Standards, Metrology, and Quality shall provide instructions on evaluating method and instruments.
6. The applicant shall cover the inspect cost and provide sufficient conditions for the inspection.
Article 20. Certificate
1. The certificate shall comply with Form No. 4.GCN in Appendix V enclosed herewith.
2. The certificate shall be valid for 05 years from the day on which it is signed.
3. The certificate shall be sent to the applicant, the Directorate for Standards, Metrology, and Quality, the Department of Standards, Metrology, and Quality of the province where the applicant is located, and shall be posted on the website of the Directorate for Standards, Metrology, and Quality.
Article 21. Document retention
1. The following documents shall be retained: The application prescribed in Article 17, inspection documents prescribed in Article 19, and the certificate prescribed in Article 20 of this Circular.
2. A set of documents shall be kept by the certifying body.
3. The facility granted the certificate (hereinafter referred to as certificate holder) shall kept a copy of the application at its headquarter or manufacturing facility to facilitate future inspections.
4. Documents shall be kept for 02 more years after they are replaced, invalidated, or expired.
Article 22. Reissuance of the certificate; adjustments to the certificate
1. At least 03 months before the expiration of the certificate or when the applicant wishes to adjust the certificate, the applicant shall compile an application for as prescribed in Article 17 of this Circular and submit it to the certifying body, whether directly or by post.
2. The certifying body shall decide whether to carry out a field inspection on a case-by-case basis.
3. The reissued or readjusted certificate shall be sent to the applicant, the Directorate for Standards, Metrology, and Quality, the Department of Standards, Metrology, and Quality of the province where the applicant is located, and shall be posted on the website of the Directorate for Standards, Metrology, and Quality.
4. The reissued certificate shall be valid for 05 years from the day on which it is signed. The expiration date of an adjusted certificate is the same as the initial certificate.
5. Documents shall be retained as prescribed in Article 21 of this Circular.
Article 23. Certificate suspension
1. The certificate shall be suspended in the cases below:
a) The certificate holder fails to sustain fulfillment of requirements in Article 10 or Article 11 of this Circular and leads to serious consequences
b) The certificate holder makes a request for suspension of the certificate.
2. The certifying body shall suspend all or part of the certificate on a case-by-case basis. The suspension shall not extend beyond 06 months from the effective date of the notification of suspension.
3. The notification of suspension shall be sent to the applicant, the Directorate for Standards, Metrology, and Quality, the Department of Standards, Metrology, and Quality of the province where the applicant is located, and shall be posted on the website of the Directorate for Standards, Metrology, and Quality.
4. After the suspension period and rectifications are made, the certificate holder may submit a dossier to request for lifting the suspension to the certifying body, whether directly or by post. The dossier consists of a written request for lifting the suspension and documents proving the rectification.
5. The certifying body shall decide whether to carry out a field inspection on a case-by-case basis as prescribed in Article 18 of this Circular.
6. The notification of lifted suspension shall be sent to the applicant, the Directorate for Standards, Metrology, and Quality, the Department of Standards, Metrology, and Quality of the province where the certificate holder is located, and shall be posted on the website of the Directorate for Standards, Metrology, and Quality.
7. The notification of suspension, lifted suspension, and relevant documents shall be retained as prescribed in Article 21 of this Circular.
Article 24. Invalidation of the certificate
1. The certificate shall be invalidated in the cases below:
a) The facility commits serious violations prescribed in Article 25 of this Circular;
b) The facility fails to rectify its violations within the suspension period written in the notification of suspension;
c) The certificate holder does not wish to keep using the true quantity marking;
d) The certificate holder goes bankrupt or is dissolved as prescribed by law.
2. The certifying body shall invalidate all or part of the certificate on a case-by-case basis.
3. The notification of invalidation shall be sent to the applicant, the Directorate for Standards, Metrology, and Quality, the Department of Standards, Metrology, and Quality of the province where the certificate holder is located, and shall be posted on the website of the Directorate for Standards, Metrology, and Quality.
Chapter V
RESPONSIBILITIES OF VARIOUS ENTITIES
Article 25. Responsibilities of entities that manufacture, import, transport, and/or sell pre-packed goods.
1. Responsibilities of entities that manufacture and/or import pre-packed goods:
a) Sustain the fulfillment of requirements in Article 10 or Article 11 of this Circular;
b) Provide truthful information about quantity of pre-packed goods;
c) Inform the customers or users of transport and storage conditions of pre-packed goods.
d) Ensure that the quantity of pre-packed goods meets technical measurement requirements in this Circular;
dd) When receiving a notification from another entity or finding that the pre-packed goods of the manufacturer or importer is not conformable with technical measurement requirements, then take remedial measures and notify competent authorities of the remedial measures and results thereof;
e) Apply for certification and sustain the use of true quantity marking on labels of pre-packed goods Group 2 in accordance with this Circular;
g) Facilitate inspections of competent authorities and persons;
h) Make a report on the use of true quantity marking on labels of pre-packed goods using form No. 3.BCTH in Appendix V enclosed herewith, then send it to the provincial Department of Standards, Metrology and Quality that received the announcement of the regulatory body that issued the certificate of eligibility to use the true quantity marking by January 31 every year or at their request.
2. Responsibilities of entities that transport and/or sell pre-packed goods:
a) Provide truthful information about quantity of pre-packed goods;
b) Inform the customers or users of transport and storage conditions of pre-packed goods.
c) Receive feedbacks of customers about inconformity of pre-packed goods with technical measurement requirements and promptly inform the manufacturer or importer;
d) Only transport and sell pre-packed goods conformable with technical measurement requirements. Pre-packed goods Group 2 must bear the true quantity marking as prescribed in this Circular;
dd) Facilitate inspections of competent authorities and persons.
Article 26. Responsibilities of Directorate for Standards, Metrology, and Quality,
1. Issue certificates of eligibility to use true quantity markings on pre-packed goods as prescribed in this Circular.
2. Provide instructions on evaluation methods and procedures; issue regulations on measurement; consider approving training programs and materials; provide training in evaluation of pre-packed goods quantity.
3. Inspect pre-packed goods quantity in accordance with regulations of law on measurements.
4. Provide professional instructions on measurement of pre-packed goods quantity; disseminate regulations of this Circular among relevant entities.
5. Inspect the receipt of announcements of the use of true quantity markings; the issuance of certificates of eligibility to use true quantity markings on pre-packed goods labels every 02 years or at the request of competent agencies.
6. Carry out inspections; handle complaints and denunciations; impose penalties for violations against the laws on measurements.
Article 27. Responsibilities of the provincial Services of Science and Technology
1. Instruct provincial Department of Standards, Metrology and Quality to inspect quantities of pre-packed goods as prescribed in Clause 3 Article 13 of the Government's Decree No. 86/2012/NĐ-CP dated October 19, 2012 and Circular No. 28/2013/TT-BKHCN dated December 17, 2013.
2. Direct the inspections of adherence to regulations of law on measurements of local entities.
Article 28. Responsibilities of provincial Department of Standards, Metrology, and Quality
1. Disseminate regulations of this Circular among relevant entities.
2. Receive announcements of use of true quantity markings; issue certificates of eligibility to use true quantity markings on pre-packed goods as prescribed in this Circular.
3. Inspect quantities of pre-packed goods in accordance with regulations of law on measurements.
4. Carry out inspections; handle complaints and denunciations; impose penalties for violations against the laws on measurements in cooperation with other entities.
5. Send a report to the Directorate for Standards, Metrology, and Quality on the receipt of announcements of the use of true quantity markings, issuance of certificates of eligibility to use true quantity markings on pre-packed goods labels, and inspections of pre-packed goods quantity.
Chapter VI
IMPLEMENTATION
Article 29. Effect
1. This Circular takes effect on August 30, 2014.
2. The following legislative documents are annulled:
a) Decision No. 02/2008/QĐ-BKHCN dated February 25, 2008 of the Minister of Science and Technology on inspection of pre-packed goods quantity;
b) Decision No. 07/2008/QĐ-BKHCN dated July 08, 2008 of the Minister of Science and Technology on List of pre-packed goods subject to state management”.
Article 30. Implementation
1. Directorate for Standards, Metrology, and Quality is responsible for providing guidance and organizing the implementation of this Circular.
2. Heads of competent authorities and relevant entities are responsible for the implementation of this Circular.
3. The difficulties that arise during the implementation must be reported to the Ministry of Science and Technology for consideration.
|
|
PP MINISTER |
APPENDIX I
MEASUREMENT
UNITS
(Promulgated together with the Circular no. 21/2014/TT-BKHCN dated July 15,
2014 of the Minister of Science and Technology)
|
Nominal quantity (Qn) |
Measurement unit |
|
|
Value |
Type of unit |
|
|
Qn < 1=""> |
Weight |
mg |
|
1 g £ Qn < 1000=""> |
g |
|
|
Qn ³ 1000 g |
kg |
|
|
Qn £ 999 mL |
Volume (liquid) |
mL (ml) or cL (cl) |
|
Qn ³ 1L |
L (l) |
|
|
Qn ≤ 1000 cm3 (1 dm3) |
Volume (solid) |
cm3, mL (ml) |
|
1 dm3 <>n < 1000="">3 |
dm3, L (l) |
|
|
Qn ³ 1000 dm3 |
m3 |
|
|
Qn < 1=""> |
Length |
mm or mm |
|
1 mm £ Qn < 100=""> |
mm or cm |
|
|
Qn ³ 100 cm |
m |
|
|
Qn < 100="">2 (1 dm2) |
Area |
mm2 or cm2 |
|
1 dm2 £ Qn < 100="">2 (1 m2) |
dm2 |
|
|
Qn ³ 1 m2 |
m2 |
|
APPENDIX II
MINIMUM
HEIGHT OF TEXT AND NUMBERS
(Promulgated together with the Circular no. 21/2014/TT-BKHCN dated July 15,
2014 of the Minister of Science and Technology)
|
Nominal quantity (Qn) |
Minimum height of text and number (mm) |
|
g or mL |
|
|
≤ 50 |
2 |
|
> 50 to 200 |
3 |
|
> 200 to 1000 |
4 |
|
kg or L |
|
|
1 |
6 |
|
Units of length, area, pcs |
2 |
APPENDIX III
CALCULATION
OF S AND COEFFICIENT K
(Promulgated together with the Circular no. 21/2014/TT-BKHCN dated July 15,
2014 of the Minister of Science and Technology)
1. Coefficient of s
Squared deviation s of real quantity is calculated using the formula below:
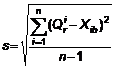
Where ![]() is
the real quantity of the ith unit of pre-packed goods (i = 1, 2,...,
n);
is
the real quantity of the ith unit of pre-packed goods (i = 1, 2,...,
n);
2. Coefficient k
The coefficient car depends on the batch size (N), sample size n, sampling place, units of measurement (unit of weight, volume, length, area, or pcs), and is determined as follows:
+ If sample of pre-packed goods is measured in a unit of weight or volume, the coefficient k shall be chosen from Table 1.
Table 1
|
Batch size (N) |
Sample size (n) |
Coefficient (k_ |
Permissible number of unconformable units of pre-packed goods (m) |
|
1 to 10 |
N |
- |
- |
|
11 to 50 |
10 |
1.028 |
0 |
|
51 to 99 |
13 |
0.848 |
1 |
|
100 to 500 |
50 |
0.379 |
3 |
|
501 to 3,200 |
80 |
0.295 |
5 |
|
> 3,200 |
125 |
0.234 |
7 |
+ If the sample of pre-packed goods is measured in a unit of length, area, or pcs, the coefficient k shall be chosen from Table 2.
Table 2
|
Batch size (N) |
Sample size (n) |
Coefficient (k) |
Permissible number of unconformable units of pre-packed goods (m) |
|
1 to 50 |
N |
- |
- |
|
26 to 50 |
3 |
1.00 |
0 |
|
51 to 150 |
5 |
0.35 |
0 |
|
151 to 500 |
8 |
0.20 |
1 |
|
501 to 3,200 |
13 |
0.15 |
1 |
|
> 3,200 |
20 |
0.10 |
1 |
+ If the sample of pre-packed goods is measured in a unit of weight or volume and the seals must be broken, the coefficient k in Table 3 shall apply.
Table 3
|
Batch size (N) |
Sample size (n) |
Coefficient (k) |
Permissible number of unconformable units of pre-packed goods (m) |
|
≥ 100 |
20 |
0.640 |
1 |
APPENDIX IV
TOLERANCE
T
(Promulgated together with the Circular no. 21/2014/TT-BKHCN dated July 15,
2014 of the Minister of Science and Technology)
|
TT |
Nominal quantity (Qn) |
Tolerance |
|
|
1 |
in g or mL |
T (1) |
|
|
in % of Qn |
in g or mL |
||
|
> 0 to 5 (2) |
- |
- |
|
|
5 to 50 |
9 |
- |
|
|
50 to 100 |
- |
4,5 |
|
|
100 to 200 |
4,5 |
- |
|
|
200 to 300 |
- |
9 |
|
|
300 to 500 |
3 |
- |
|
|
500 to 1,000 |
- |
15 |
|
|
1,000 to 10,000 |
1,5 |
- |
|
|
10,000 to 15,000 |
- |
150 |
|
|
> 15,000 |
1,0 |
- |
|
|
2 |
in m |
in % of Qn |
|
|
Qn £ 5 |
Unconformable units of pre-packed goods not allowed |
||
|
Qn > 5 |
2 |
||
|
3 |
in m2 |
in % of Qn |
|
|
All Qn |
3 |
||
|
4 |
in pcs |
in % of Qn |
|
|
Qn £ 50 |
Unconformable units of pre-packed goods not allowed |
||
|
Qn > 50 |
1(3) |
||
Notes:
(1): in section 1, T may be rounded to the nearest decimal fraction of g or mL if Qn £ 1000 g (or 1000 mL) or to the nearest unit of g or mL if Qn > 1000 g (or 1000 mL);
(2): only Xtb has to be satisfactory
(3): T is rounded to the nearest integer.






