Nội dung toàn văn Decision 478/QD-QLD 2021 fuidelines for transporting preserving and using COVID19 vaccine
|
MINISTRY OF
HEALTH |
SOCIALIST
REPUBLIC OF VIETNAM |
|
No. 478/QD-QLD |
Hanoi, August 10, 2021 |
DECISION
GUIDELINES FOR RECEIVING, TRANSPORTING, PRESERVING, AND USING COVID-19 VACCINE
DIRECTOR OF DRUG ADMINISTRATION OF VIETNAM
Pursuant to Decree No. 75/2017/ND-CP dated June 20, 2017 of Government on functions, tasks, powers, and organizational structure of Ministry of Health;
Pursuant to Decision No. 7868/QD-BYT dated December 28, 2018 of Minister of Health on functions, tasks, powers and organizational structure of Drug Administration of Vietnam affiliated to Minister of Health;
Pursuant to Decision No. 3043/QD-BYT dated June 24, 2021 of Minister of Health establishing Steering Committee for Nationwide Launching of COVID-19 Vaccination Campaign;
Pursuant to Circular No. 03/2018/TT-BYT dated February 9, 2018 of Minister of Health on Good practice of drug and pharmaceutical starting material distribution;
Pursuant to Circular No. 36/2018/TT-BYT dated November 22, 2018 of Minister of Health on Good practice of drug and pharmaceutical starting material preservation;
At request of Director of Pharmaceutical Trade Management Department and Director of Drug Quality Control Department.
HEREBY DECIDES:
Article 1. Issue Guidelines on receiving, transporting, preserving, and using COVID-19 vaccine in Nationwide COVID-19 vaccination campaign under Annex attached hereto.
Article 2. Heads of relevant agencies and entities shall rely on these guidelines to develop and issue specialized procedures applicable to activities in agencies and entities.
Article 3. This Decision comes into effect from the day of signing.
Article 4. Chief of Office, Director of Pharmaceutical Trade Management Department, Director of Drug Quality Control Department - Drug Administration of Vietnam, Director of National Institute of Hygiene and Epidemiology, and heads of relevant agencies and entities are responsible for implementation of this Decision./.
|
|
PP. DIRECTOR |
ANNEX
GUIDELINES FOR
RECEIVING, TRANSPORTING, PRESERVING, AND USING COVID-19 VACCINE IN NATIONWIDE
COVID-19 VACCINATION CAMPAIGN
(Attached to Decision No. 478/QD-QLD dated August 10, 2021 of Director
of Drug Administration of Vietnam)
|
No. |
Guidelines |
|
1 |
Receiving vaccine |
|
2 |
Preserving vaccine in refrigerators |
|
3 |
Preserving diluents |
|
4 |
Preserving vaccine in ultra-low freezers |
|
5 |
Preserving vaccine in ultra-low freeze temperature in thermal containers |
|
6 |
Monitoring vaccine preservation temperature |
|
7 |
Frosting and defrosting ice-packs |
|
8 |
Packing vaccine in cold boxes |
|
9 |
Transporting vaccine with refrigerated trucks |
|
10 |
Transporting vaccine with cold boxes |
|
11 |
Maintaining refrigerators |
|
12 |
Responding to emergencies |
|
13 |
Allocating and distributing vaccine |
|
14 |
Checking vaccine and diluents |
|
15 |
Recalling and storing vaccine separately |
|
16 |
Responding to issues during transportation |
|
17 |
Transporting COVID-19 vaccine from airports to storage facilities |
|
18 |
Returning vaccine containers to manufacturers |
GUIDELINES ON RECEIVING VACCINE AND DILUENTS
1. Objectives
These guidelines aim to assist personnel in agreeing and complying with steps for receiving vaccine and diluents safely while reducing impact of the surrounding (temperature, light, etc.) on vaccine and diluents, and avoiding confusion.
2. Equipment and relevant documents
Cold chain equipment and temperature monitoring instrument consist of: refrigerators, cold boxes, ice-packs, temperature monitoring instrument.
Documents on vaccine distribution, vaccine logbook, records of delivery/goods-dispatch note, etc.
3. Procedures
3.1. Preparation
3.1.1. Preparing cold equipment with adequate volume to contain vaccine prior to receipt.
- Refrigerators: for preservation at +2oC to +8oC.
- In case refrigerators are containing vaccine, storage personnel shall rearrange said vaccine to make adequate volume and separate to prepare for new vaccine.
3.1.2. Examining and preparing temperature monitoring instrument for each cold equipment: Examine working conditions of temperature monitoring instrument of each refrigerator and automatic temperature reading instrument,
3.1.3. Preparing vaccine logbook to record information upon receiving vaccine.
3.1.4. Washing hands: Personnel must wash hands before holding vaccine containers and vials.
3.2. Implementation steps
|
No. |
Work entry |
Assignment |
|
1 |
Transfer relevant documents: goods-dispatch note/record of delivery, permit for dispatching received vaccine |
Storage personnel Vaccine receiving personnel Vaccine delivering personnel |
|
2 |
Examine preservation temperature when receiving vaccine (temperature of refrigerated trucks, temperature of each cold box serving vaccine transportation). Specify temperature in record of delivery |
Storage personnel Vaccine receiving personnel Vaccine delivering personnel |
|
3 |
Transfer vaccine: Examine and cross check each vaccine and diluent with goods-dispatch note: * Visual properties: Examine conditions of transport vehicles/cold-boxes: external conditions, hygiene, arrangement, Examine conditions of each vaccine container/box: integrity, contamination, etc. * In details: Cross check with relevant documents: - Name of vaccine/diluent - Batch number, expiry date. - Number of vaccine of each batch - Conditions of temperature monitoring instrument, - Temperature of each cold-box serving vaccine transportation - Time and date of receiving and inspecting each cold-box serving vaccine preservation Any irregularity or discrepancy between actual conditions and those specified in notice of dispatch (example: contaminated, damaged, blurred packaging, inadequate number of vaccine, batch number, expiry date, etc.) must be reported to superiors for timely solutions |
Storage personnel Vaccine receiving personnel Vaccine delivering personnel |
|
4 |
Arrange vaccine in cold equipment according to guidelines on preserving vaccine refrigerators |
Storage personnel |
|
5 |
Arrange diluent in shelves/racks in storage |
Storage personnel |
|
6 |
Sign record of delivery/goods-dispatch note and store in documents on receiving vaccine/diluent. |
Storage personnel |
|
7 |
Record vaccine and diluent information in “Receive” section in vaccine logbook: name of delivering entities, type of vaccine, diluent, place of manufacturing, quantity, batch number, expiry date, temperature/conditions of vaccine vial monitor (VVM) (if any), visual conditions, etc. |
Storage personnel |
GUIDELINES ON PRESERVING VACCINE IN REFRIGERATORS
1. Objectives
These guidelines aim to assist storage personnel and other personnel engaging in preservation of vaccine/diluent in arranging, preserving vaccine/diluent safely in refrigerators as per the law, and reducing error and impact of temperature, light, etc. on vaccine quality.
2. Equipment and relevant documents
- Refrigerators.
- Temperature monitoring instrument: thermometer, automatic temperature reading equipment, freeze tag (if any).
- Daily temperature graph.
3. Procedures
3.1. General principles:
- DO NOT place drugs, chemicals, specimen, food, and drinks in vaccine refrigerators.
- DO NOT preserve expired vaccine, vaccine with labels covered in mold in vaccine refrigerators with other vaccine.
- DO NOT open refrigerators regularly or unnecessarily. List of vaccine in preservation must be posted on the outside of the refrigerators.
3.2. Washing hands: Personnel must wash hands before holding vaccine containers and vials.
3.3. Implementation steps
|
No. |
Entry |
Assignment |
|
1. |
Place vaccine containers in the basket of a refrigerator. NEVER remove the basket of a refrigerator to allow more storage capacity. Leave a 2 cm gap between vaccine containers to allow cold air to flow. |
Storage personnel Personnel arranging and preserving vaccine |
|
2. |
Arrange vaccine by type, batch, and expiry date to distribute with ease while prioritizing vaccine closer to its expiration date and vaccine preserved earlier than the others. Physical separation must be installed between different types of vaccine stored in the same refrigerator. Containers of different batches of vaccine must be marked and separated. Vaccine that is received at a later date but is closer to expiration date shall be prioritized for distribution based on expiration date. |
Storage personnel Personnel arranging and preserving vaccine |
|
3. |
Place temperature monitoring instrument and vaccine in designated position. |
Storage personnel Personnel arranging and preserving vaccine |
|
4. |
Examine and monitor temperature of vaccine refrigerators on a daily basis and record temperature in temperature log according to guidelines on monitoring and recording vaccine preservation temperature. |
Storage personnel Personnel arranging and preserving vaccine |
GUIDELINES ON PRESERVING DILUENTS
1. Objectives
Diluents must be preserved in appropriate temperature, at room temperature (≤ 25 °C) or from +2 oC to +8 oC. These guidelines aim to instruct storage personnel and other personnel engaging in preservation of diluents on how to arrange and preserve diluents safely as per the law in order to reduce all risks affecting diluent quality.
2. Equipment and relevant documents
- Refrigerators.
- Diluent preservation shelves/racks.
- Daily temperature graph.
3. Procedures
3.1. General principles:
- Diluents must be preserved in dry places and away from surfaces with risks of mold and moisture.
- Diluents are arranged on shelves and racks; DO NOT place diluents close to the walls or to the ground.
- Diluents may be preserved in room temperature (≤25 °C) and must be preserved in in temperature ranging from +2 oC to +8 oC for 24 hours before being reconstituted for vaccine.
- DO NOT let diluents get frosted.
3.2. Washing hands: Personnel must wash hands before holding vaccine containers and vials.
3.3. Preserving diluents at room temperature (≤25°C):
|
No. |
Entry |
Assignment |
|
1. |
Place diluent containers on shelves or racks while maintaining a 2 cm space between any 2 containers for air flow. DO NOT place diluent containers close to the wall or close to the ground Individual diluent vials must be placed on small labeled containers whose labels contain information on name, number, expiry date; individual diluent vials of different batches must not be placed in the same containers |
Storage personnel Personnel arranging and preserving vaccine |
|
2. |
Arrange diluents by type and batch in order to avoid confusion during distribution along vaccine. Do not place diluent containers of different batches on the same shelves or racks, if such a case is inevitable, place on shelves or racks where physical separation is installed and distinctively marked |
Storage personnel Personnel arranging and preserving vaccine |
|
3. |
At least 24 hours before reconstitution, diluents must be preserved in a temperature ranging from +2 oC to +8 oC While preserving diluents in refrigerators, diluents must not be stored together with vaccine and must be separated, marked in order to prevent confusion. |
Storage personnel Personnel arranging and preserving vaccine |
GUIDELINES ON PRESERVING VACCINE IN ULTRA-LOW FREEZERS
1. Objectives
These guidelines aim to instruct storage personnel on how to preserve vaccine in ultra-low freezers in a manner than ensures safety for the vaccine and reduce effect of elements that diminish vaccine effect and safety of storage keepers. .
2. Equipment and relevant documents
- Protective equipment: Coat + hat, masks, goggles, thermal and watertight gloves, and boots.
- Ultra-low freezers.
3. Terminology – acronyms
- FIFO: Distribution principle of “First-In-First-Out”
- FEFO: Distribution principle of “First-Expiry-First-Out”
- Safety area: refers to positions in a ultra-low freezer with temperature ranging from -80 oC to -60 oC, determined after temperature mapping of each cold compartment has been conducted.
4. Steps of implementation
4.1. Human safety assurance:
+ At least 2 personnel must be present when receiving, arranging, and preserving vaccine in ultra-low freeze utilizing dry ice or ultra-low freezers
+ Prior to opening Pfizer thermal vaccine containers utilizing dry ice (CO2) and employing ultra-low freezers, personnel must wear warm clothes, masks, thick and watertight gloves, goggles, boots.
+ Do not make direct contact with the dry ice and/or trays, walls of the ultra-low freezers.
+ Do not inhale cold air emitting from dry ice and the ultra-low freezers.
Safety remarks: First aid upon any incident involving dry ice:
• Inhaling CO2: Any breathing difficulty or headache can be symptoms of inhaling too much CO2. Immediately move to areas with fresh air. Provide medical care and consulting at the facility immediately (e.g. clear the air way, rest with elevated head position, breathe oxygen, etc.)
Swallowing: Rinse mouth with clean water immediately if possible. Do not insert any object into the mouth of an unconscious person. Do not induce vomit unless otherwise instructed by medical personnel. Call emergency medical response in case of any physical injury.
• Eye contact: Rinse eyes with lots of water for at least 15 minutes, lift the upper and lower eyelids to avoid any substance from sticking to them. Bring into emergency medical aid immediately, etc.
• Skin contact: Take off contaminated clothes (if possible), wash the contact area with great amount of water to defrost. May employ soap to wash, do not rub affected area. Call emergency medical response in case of suspected physical injury, etc.
4.2. Safe vaccine preservation: Warehouse personnel shall implement
4.2.1. If vaccine is transported in ultra-low free conditions (un-defrosted vaccine)
a. Immediately after opening thermal containers carrying vaccine to inspect and receive, open the door of ultra-low freezers to place vaccine trays into compartments of the ultra-low freezers. Note that every arrangement only fills up to 3/4 of total preservation volume of a ultra-low freezer.
b. Doors of a ultra-low freezer must not remain open for more than 3 minutes or a siren must be issued when temperature of the freezer rises to -60 oC.
c. Arrange vaccine containers by batch and expiry date to enable inspection and distribution conforming to FIFO and FEFO principles and VVM display. Leave a 5-10 cm vertical space to differentiate vaccine batches/types and allow airflow. Ensure visibility of labels of containers.
d. Tag each container of vaccine/diluent batch (under Annex attached thereto)
e. Individual containers must be placed in trays or holders.
f. Remove unqualified (expired) vaccine vials/containers from the freezers and store in isolated containment area awaiting disposal.
4.2.2. If vaccine is transported in temperature ranging from +2 oC to +8 oC (defrosted vaccine): arrange vaccine in refrigerators as per procedures.
4.3. Monitor and control of vaccine preservation temperature: Warehouse personnel shall implement
- Inspect temperature displayed on monitor of refrigerators twice per day (in the morning upon arrival and in the afternoon before leaving for 7 days/week).
- Record the temperature in monitor schedule (Annex 1: Ultra-low freezer temperature monitoring schedule).
- At the end of every week, specify feedback and include in file for storage.
- On a monthly basis, use a flash drive to copy and store data on vaccine preservation temperature extracted from temperature monitoring instrument of each refrigerator.
4.4. Record and storage of vaccine management dossiers: Warehouse personnel shall implement
- Each refrigerator must include a storage manifest that specifies batch, expiry date, time and place of receipt/distribution, amount of input/output/stock (storage manifest model) and graph of positions of vaccine stored in the refrigerators (Annex 4) to serve easy distribution and avoid searching for vaccine in opened refrigerators.
- Record information on vaccine in preservation and distribution in logbook
- Check vaccine on a monthly basis and keep records thereof. Specify physical inspection results of each vaccine batch and keep records thereof.
Annex 1: ULTRA-LOW FREEZER TEMPERATURE MONITORING SCHEDULE
From ………. (date) to ............ (date)
Ultra-low freezer No.
|
Date |
|
|
|
|
|
|
|
|||||||
|
Temperature (°C) |
S |
C |
S |
C |
S |
C |
S |
C |
S |
C |
S |
C |
S |
C |
|
-30 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-55 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-61 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-62 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-63 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-64 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-65 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-66 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-67 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-68 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-69 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-72 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-73 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-74 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-75 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-76 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-77 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-78 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-79 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-80 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-82 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-84 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-86 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-88 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-90 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: S- Temperature recorded in the morning (from 8 a.m. to 9 a.m.)
C- Temperature recorded in the afternoon (from 4 p.m. to 5 p.m.)
FEEDBACK
|
Time |
Temperature range |
Remedial measures |
|
|
|
|
|
|
|
|
Note: + Record irregular temperature range, reason and remedial measures thereof.
+ In case of no irregular temperature range: write “Operational freezer”
Entity: …………………
STORAGE MANIFEST
Name of vaccine: …………………………………………………………………………
Batch: …………………………… Expiry date: ………………………
From …………… (date) to …………… (date)
|
Time |
Entry |
Quantity (dose) |
||
|
Input |
Output |
Stock |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supervision |
Storage personnel (signature) |
GRAPH OF VACCINE PRESERVED IN ULTRA-LOW FREEZER ………
From …………… (date) to …………… (date)
|
FREEZER COMPARTMENT |
NAME OF VACCINE |
BATCH |
QUANTITY (dose) |
|
1 |
|
|
|
|
2 |
|
|
|
|
3 |
|
|
|
|
4 |
|
|
|
Storage personnel (signature)
GUIDELINES ON PRESERVING VACCINE IN ULTRA-LOW FREEZE TEMPERATURE IN PFIZER’S THERMAL CONTAINERS
1. Objectives
These guidelines aim to guide steps for adding dry ice in specialized thermal containers for preserving vaccine in ultra-low freeze temperature in a manner that guarantees safety for humans and vaccine.
2. Equipment and relevant documents
- Protective equipment: Coat + hat, masks, goggles, thermal and watertight gloves, and boots.
- 1*2 cm dry ice: purchased 24 hours in advance to ensure sufficient dry ice for any additional demand (approximately 10-15 kg/container)
- Dry ice handling equipment: Plastic shovel/scoop
- Pfizer BioNTech provides 2 types of specialized thermal containers for preserving and transporting vaccine: SOFTBOX and AEROSAFE.
3. Procedures for adding dry ice when preserving vaccine over long period of time in ultra-low freeze temperature with Pfizer’s specialized thermal containers
IMPORTANT NOTE when using specialized thermal containers to preserve vaccine in ultra-low freeze temperature:
- Preserve thermal containers in room temperature of 15 oC to 25 oC to maintain their ability preserve ULTRA-LOW FREEZE temperature.
- In order to maintain level of dry ice and ultra-low freeze temperature of vaccine, proceed as follows:
Ø 2x/day: Do not open thermal containers for transporting vaccine more than 2 times per day
Ø 3 minutes: Do not open thermal containers for transporting vaccine more than 2 minutes each time
Ø 5 days: Add more dry ice to thermal containers for transporting vaccine once every 5 days
- If lids of transport containers must be opened multiple times per day, add dry ice more regularly.
- All accessories that come with the thermal containers must be kept in the containers with care when returning the containers to the manufacturers for recycling.
3.1. Preparation prior to receiving/when adding dry ice to thermal containers
- Assign at least 2 employees to perform the task
- Employees who interact with dry ice must wear protective clothing: long-sleeved warm clothes, boots, goggles, gloves, and masks to avoid frostbite
- Open door for natural ventilation/turn on ventilating mechanisms in areas where thermal containers are preserved, dry ice is added to avoid CO2 poisoning.
- Place warning signs in areas where dry ice is added to containers: CẢNH BÁO: DẢM BẢO AN TOÀN TIẾP XÚC DÁ KHÔ (WARNING: ENSURE SAFETY WHEN INTERACTING WITH DRY ICE)
3.2. Within the first 24 hours after receiving vaccine: open the containers, examine and add more dry ice to the thermal containers.
3.3. Procedures for adding more dry ice
3.3.1. Place thermal containers where more dry ice is to be added down the pallet
Place a container down the pallet (without direct contact with the floor) when that container is to be added with more dry ice.
3.3.2. Procedures for adding dry ice to a SOFTBOX thermal container
3.2. Within the first 24 hours after receiving vaccine: open the containers, examine and add more dry ice to the thermal containers.
3.3. Procedures for adding more dry ice
3.3.1. Place the thermal container where more dry ice is to be added down the pallet
Place the container down the pallet (without direct contact with the floor) when that container is to be added with more dry ice.
3.3.2. Procedures for adding dry ice to a SOFTBOX thermal container
|
1. Note on components of a thermal container to allow the addition of dry ice to be implemented in the fastest time (3 minutes for each container) Thermal foam lid is attached to the lid of the cardboard container and installed with temperature monitoring equipment The box that holds vaccine trays must be placed in the container after use in order to be returned to manufacturers |
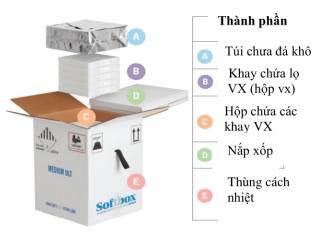
|
||||||||||||
|
2. Open the thermal container (E) by cutting the outside tape, place 3 fingers into the gap in order to lift the foam lid attached to the container lid (D) Do not tear the shell or damage the temperature monitoring wire |
|
||||||||||||
|
3. Use gloves to lift the dry ice pod (A) and place down the pallet |
|
||||||||||||
|
4. Use scoop to move dry ice into the compartment surrounding the box that holds vaccine trays (C) until dry ice level equals the top of the box that holds vaccine trays |
|
||||||||||||
|
Place dry ice pod (A) on top of the box that holds vaccine trays. Open (A) gently and add more dry ice while spreading them evenly (do not fill up too much). |
|
||||||||||||
|
6. Close the dry ice pod (A) to its original state in a manner that the pod fits the compartment and combines with the topmost edge of the thermal container to create a flat surface |
|
||||||||||||
|
7. Place the foam lid (D) and cardboard lid of the thermal container (E) and seal with tape. Preserve the container in airy places under 25 oC |
|
3.3.3. Procedures for adding more dry ice to an AEROSAFE thermal container
|
1. Note on components of a thermal container to allow the addition of dry ice to be implemented in the fastest time (3 minutes for each container) The foam lid is attached to the cardboard container with a thin aluminum strip and installed with a temperature monitoring instrument which must not be damaged. Dry ice pod must be returned to the container when returning the container to the manufacturers |
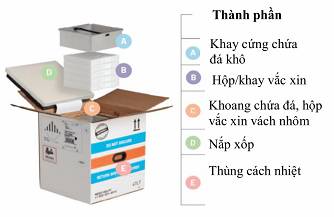
|
||||||||||||
|
2. Open the thermal container (E) by cutting the outside tape; open the lid of the cardboard container. Use gloves to lift the thermal foam lid (D) by grasping the gap while trying not to damage the aluminum strip or the temperature monitoring wire |
|
||||||||||||
|
3. Use gloves to grip the harness, lift the dry ice pod (A), and place down next to the container |
|
||||||||||||
|
4. Use scoop to move dry ice into the compartment surrounding the container until dry ice fills the top edge of vaccine compartment |
|
||||||||||||
|
5. Place the dry ice pod (A) fit into the vaccine compartment then add more dry ice to the pod (A) without filling up too much. |
|
||||||||||||
|
6. Place the foam lid (D) and cardboard lid of the thermal container (E) and seal with tape. Preserve the container in airy places under 25 oC. |
|
Schedule for monitoring dry ice addition of thermal container No.
From ………. (date) to ............ (date)
|
No. |
Time |
Date |
Individual in charge (Signature/name) |
Additional amount of dry ice (estimated) |
Supervisor |
|
1 |
|
|
|
.?. …… kg |
|
|
2 |
|
|
|
|
|
|
3 |
|
|
|
|
|
|
4 |
|
|
|
|
|
|
5 |
|
|
|
|
|
|
6 |
|
|
|
|
|
(Store documents at the entity)
GUIDELINES ON MONITORING AND RECORDING VACCINE PRESERVATION TEMPERATURE
1. Objectives
Vaccine must be preserved at appropriate temperature in order to avoid spoilage. These guidelines aim to list activities and requirements for monitoring and recording daily temperature during vaccine preservation to respond in a timely manner when temperature is outside of permissible range in order to ensure safety and quality of vaccine.
2. Equipment and relevant documents
- Daily temperature graph.
- Temperature monitoring instrument: thermometer, automatic temperature reading equipment, freeze tag.
3. Procedures
|
No. |
Work entry |
Assignment |
|
|
1. Preparation |
1.1 |
Place 1 automatic temperature recording equipment in each cold equipment that preserves vaccine. Place 1 freeze tag (if any) together with vaccine that can easily be spoiled by freezing temperature. Do not place in compartments with negative temperature. Equipment must be coded (numbered) based on cold equipment (refrigerators) |
Storage personnel Officials in charge of monitoring and preserving vaccine |
|
|
Installation location of temperature monitoring instrument: - For thermometer and automatic temperature recording instrument: place together with vaccine in a position that allows easy tracking and is susceptible to change in temperature caused by opening and closing the refrigerators. - For freeze tags: place together with vaccine that is susceptible to freezing temperature. Do not place in compartments with negative temperature. |
Storage personnel Officials in charge of monitoring and preserving vaccine |
|
|
1.2 |
Specify number of each cold equipment in daily graph for monitoring temperature. |
Storage personnel |
|
|
2. Daily activities |
2.1 |
Examine reading of automatic temperature recording instrument and write down the monitoring graph once in the morning (upon arrival) and once at the end of the afternoon (before leaving) for 7 days a week, including Saturday, Sunday, and holidays. |
Storage personnel Officials in charge of monitoring and preserving vaccine |
|
2.2 |
Examine vaccine vial monitor (VVM) and freeze tag (if any). Upon detecting any irregularity, report to superior/supervisor to comply with procedures for responding to incidents in vaccine preservation. |
Storage personnel |
|
|
2.3 |
Examine daily temperature range of automatic temperature recording instrument every 24 hours and record the highest temperature and the lowest temperature in graph for monitoring temperature. Daily temperature must be within the permissible range as per the law. |
Storage personnel Officials in charge of monitoring and preserving vaccine |
|
|
2.4 |
If recorded temperature is outside of permissible range, report to superior/supervisor and comply with procedures for responding to incidents in preserving vaccine. |
Officials in charge of monitoring and preserving vaccine Storage personnel Officials in charge of vaccine storage specialty |
|
|
3. Monthly activities |
3.1 |
At the end of each month, assess and comment on temperature during preservation period of each cold equipment during the month; supervisor shall sign the graph for monitoring temperature. |
Vaccine storage management personnel. Officials in charge of vaccine storage specialty |
|
3.2 |
Change to graph for monitoring temperature for the next month and store graph of the previous month. Specify all information in the graph for monitoring temperature (number of cold equipment, initial and final date of supervision, etc.). |
Vaccine storage management personnel |
|
|
3.3 |
Print/back up temperature data from automatic temperature recording instrument of each cold equipment. |
Vaccine storage management personnel |
|
|
3.4 |
Report to superior if vaccine temperature of cold equipment in the month is unstable. |
Vaccine storage management personnel Officials in charge of vaccine storage specialty |
|
GUIDELINES ON FROSTING AND DEFROSTING ICE-PACKS
1. Objectives
These guidelines aim to provide instructions on how to frost and defrost ice-packs appropriately in order to preserve vaccine safely and properly. Inappropriately defrosted ice-packs may affect vaccine as it exposes vaccine to freezing temperature.
2. Equipment and relevant documents
- Defrosting cabinets for ice-packs or refrigerators with freezer compartment.
- Ice-packs.
- Clean towel.
3. Procedures
|
No. |
Entry |
Assignment |
|
1. |
Calculate demand for ice-packs: - Rely on plans for preserving and transporting vaccine using cold boxes to prepare adequate number of ice-packs. Note that dimension of ice-packs must suit types of cold boxes. - Calculate time necessary for defrosting the adequate amount of ice-packs (minimum time for defrosting ice-packs is 24 hours). |
Storage personnel |
|
2. |
Frosting ice-packs: - Fill ice-packs with clean water until designated mark. Seal the lid tight. Hold the ice-packs upside-down and shake to ensure that the ice-packs are sealed. - Arrange the ice-packs in standing positions or tilted positions in freezer compartment for at least 24 hours to completely freeze water in the ice-packs. - Collect frozen ice-packs out of freezer compartments and close the freezer’s doors. |
Storage personnel Assigned storage personnel |
|
3. |
Defrosting ice-packs: - Place frosted ice-packs in ambient temperature for 15-30 minutes (depending on ambient temperature) until parts of the ice start to melt. - Inspect by shaking the ice-packs until “sloshing” sound is heard. - Use clean towel to clean defrosted ice-packs before placing them into cold boxes/vaccine carriers. |
Storage personnel |
GUIDELINES ON PACKING VACCINE IN COLD BOXES
1. Objectives
These guidelines aim to provide steps for packing vaccine in cold boxes with properly defrosted ice-packs to reduce impact of temperature on vaccine quality during transportation and preservation.
2. Equipment and relevant documents
Cold boxes, ice-packs, temperature monitoring instrument
3. Procedures
3.1. Preparation:
3.1.1. Preparing ice-packs: comply with procedures for frosting and defrosting ice-packs (Procedure No. 6).
3.1.2. Prepare cold boxes: cold boxes must be clean and dry before containing vaccine
3.1.3. Washing hands: Personnel must wash hands before holding vaccine containers and vials.
3.2. Packing vaccine in cold boxes, using defrosted ice-packs:
|
No. |
Entry |
Assignment |
|
1. |
Place defrosted ice-packs in 4 surrounding walls of the cold boxes (number of ice-packs and packaging methods shall conform to use instruction manuals of each type of cold box). |
Storage personnel and vaccine transporters Specialized personnel of storage |
|
2. |
Individual vaccine and diluent vials must be packed in covered containers. |
Vaccine storage employees and vaccine transporters |
|
3. |
Place vaccine and diluent containers in the middle of cold boxes in a manner that vaccine vials are oriented upwards. Note to wedge vaccine containers tightly to avoid collision during transportation. |
Storage personnel and vaccine transporters |
|
4. |
Place temperature monitoring instrument close to the vaccine. These equipment must avoid direct contact with ice-packs |
Storage personnel and vaccine transporters |
|
5. |
Place defrosted ice-packs on top. |
Storage personnel and vaccine transporters |
|
6. |
Close cold boxes and lock. |
Storage personnel and vaccine transporters |
|
7. |
Do not leave cold boxes directly under sunlight during transportation. |
Vaccine transporters |
GUIDELINES ON TRANSPORTING VACCINE WITH REFRIGERATED TRUCKS
1. Objectives
These guidelines aim to provide instructions on how to transport vaccine using refrigerated trucks from vaccine storage in districts to storage of other entities in such a manner that reduces errors and elements affecting vaccine quality during transportation while providing safe and quality vaccine to facilities promptly and adequately.
2. Equipment and relevant documents
- Official Dispatch/Decision on distributing vaccine.
- Record of delivery, goods-dispatch note, certificate of vaccine dispatch. (Annex 1,2, and 3)
- Temperature monitoring instrument.
- Refrigerated trucks
- Information on recipient: Address, phone number of personnel in charge of receiving facility or superior in charge of receiving facility
3. Procedures
Step 1: Producing vaccine transportation plan
Superior in charge of vaccine storage and personnel in charge of vaccine storage shall:
- Produce plans for distributing and transporting vaccine with specific quantity, type, batch of vaccine, etc. and estimated time of delivery to receiving facility. Notify receiving facility about transport plans (estimated time of vaccine delivery, number and type of vaccine)
- Dispatch automobiles and assign drivers as per prepared plan.
- Inform drivers:
+ Expected route, time, location of delivery, full name and phone number of personnel in charge at receiving facility.
+ Prior to delivery date, inspect technical conditions of vehicles such as tires, brake, gasoline, oil, machinery, refrigeration system.
+ 1 – 2 hours prior to loading vaccine onto the vehicles, start refrigeration system to bring temperature in cargo compartment down to from +2 oC to +8 oC.
+ Instructions on responding to incidents during transport must be present on refrigerated trucks at all time. (Procedure No. 15).
Step 2: Packing vaccine and diluents, and preparing temperature monitoring instrument
Storage personnel, vaccine storage assistant/transport personnel shall:
- Prepare vaccine transfer documents as per Procedure No. 12: Vaccine allocation and distribution
- Check and cross check type of vaccine, diluents (quantity, batch number, expiry date, etc.) in practice and those in goods-dispatch note. If remaining number of vaccine in practice in store does not match with number of vaccine according to batch/expiry date on goods-dispatch note, storage keepers must report to superiors of storage to revise and produce another dispatch note/record.
- Prior to packing, examine and record preservation temperature and vaccine conditions (visual properties of labels, humidity, mold, color) in record of vaccine transfer (according to Annex 1).
- Vaccine shall be packed in turns and arranged in cardboard boxes based on vaccine type. Specify name of recipient, type of vaccine, quantity, batch number, and expiry date on the outside of vaccine containers/boxes. After packing, check again according to dispatch note and seal each vaccine container/box (storage personnel shall be responsible for quantity of vaccine).
- Install temperature monitoring instrument.
Step 3: Loading vaccine onto refrigerated trucks and transporting
Storage personnel, vaccine storage assistant/transport personnel, and drivers shall:
- Examine temperature of cargo compartments of refrigerated trucks and only load vaccine onto the trucks when temperature is between +2 oC and +8 oC.
- Immediately load vaccine containers/boxes of each receiving facility onto refrigerated trucks while leaving adequate space between any 2 containers/boxes for cold air; do not place vaccine susceptible to freezing temperature close to refrigerating unit (place at least 0.5 meters away from refrigerating unit); stack vaccine containers/boxes no higher than 2/3 of the height of cargo compartment; arrange containers/boxes in order and firmly to avoid collision, falling, and in a manner that allows easy distribution depending on expected route (containers/boxes to be distributed to the first receiving facility shall be placed in the outermost position).
- Place temperature monitoring instrument in vaccine compartment of refrigerated trucks. Lock the door leading to cargo compartments with care and immediately transport vaccine to receiving facility as per itinerary.
- Sign vaccine delivery log (Annex 2), storage personnel shall transfer relevant documents to transport personnel (goods-dispatch note, record of delivery, dispatch permit of each vaccine batch) and temperature monitoring instrument.
Transport personnel and drivers shall:
- Monitor temperature in refrigerated compartment during transportation (via temperature monitoring system in the cabin) and maintain the temperature between +2 oC and +8 oC. If temperature of cargo compartment is not within +2 oC to +8 oC, comply with “Guidelines on responding to incidents during vaccine transport using refrigerated trucks” (Procedures No. 15).
- During any rest along the route, keep the refrigerating system of the trucks functional at all time (by keeping the engine on or tapping into a power source). In case of overnight rest, monitor temperature of refrigerated compartment once every 2-3 hours.
- 45-60 minutes prior to arriving at vaccine delivery location, contact respective storage personnel to cooperate in receiving vaccine.
Step 4: Transferring vaccine
- Upon arrival, transport personnel shall immediately transfer vaccine and relevant documents (goods-dispatch note, record of delivery, dispatch permit) to storage personnel of receiving facility.
- Cooperate with storage personnel in examining and recording temperature of refrigerated compartment and temperature reading on temperature recording instrument in record of delivery before transferring vaccine.
- Transfer adequate number of vaccine containers/boxes to receiving facility.
- Cooperate with storage personnel of receiving facility in inspecting quantity, batch, expiry date of vaccine and diluents delivered.
- Any discrepancy in terms of quantity, batch, or expiry date of vaccine/diluents found by storage personnel of receiving facility must be reported to storage personnel of distributing facility or superior of storage facility for solution.
- Fill in all information on time of transfer and conditions of vaccine in record of transfer. Collect signature and seal of superior of receiving storage facility.
- If any irregularity is found during transfer process, receiving storage facility has the right to reject the vaccine. In such case, receiving storage personnel must produce record and state reason for rejection, request their superior to confirm, and immediately inform superior of distributing storage facility for timely solutions.
Step 5: Transferring equipment, documents, and backing up data on vaccine temperature during transportation
- After returning to distributing storage facility, transport personnel are responsible for immediately transferring thermometer, record of delivery/goods-dispatch note of the delivery to storage personnel.
- Storage personnel shall distribute, print, and back up temperature data recorded by transport personnel from temperature monitoring instrument on refrigerated trucks and thermometer installed in refrigerated compartments while examining temperature data during transportation. If vaccine temperature reading during transportation is not satisfactory to manufacturer’s requirements (i.e. situating outside of +2 oC to +8 oC), transport personnel and storage personnel must acknowledge the situation, report, and clarify the cause.
- At the end of each delivery session, storage personnel shall submit reports to heads of military region.
Form:
SOCIALIST REPUBLIC
OF VIETNAM
Independence – Freedom – Happiness
----------------
RECORD OF VACCINE DELIVERY
Vaccine deliverer:
- Full name of representative: ………………………. Titles:
- Full name of deliverer: ………………………. Titles:
Vaccine recipient:
- Full name of representative: ………………………. Titles:
- Full name of recipient: ………………………. Titles:
At ………, …………………… (time and date), the parties proceed to transfer vaccine as follows:
Part I: Documents on vaccine transfer:
|
|
Number of Official Dispatch on distribution of vaccine |
Date of signing Official Dispatch |
Permit of National Control Institute |
Goods-dispatch Note |
|
Before receiving |
|
|
Yes □ No □ |
|
|
Upon receiving |
|
|
Yes □ No □ |
Yes □ No □ |
Part II: Details on received vaccine and diluents:
|
Name of vaccine |
Unit |
Quantity |
Number of batch |
Expiry date |
Manufacturer |
Manufacturing country |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluent |
Unit |
Quantity |
Number of batch |
Expiry date |
Manufacturer |
Manufacturing country |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Part III. Conditions of temperature monitoring instrument during transport
|
Total examined cargoes: |
Vaccine temperature: oC |
|||
|
Temperature monitoring instrument: |
VVM □ (stage 1, 2, 3, and 4) |
Freeze tags □ (write X or V) |
LogTag device Yes □ No □ |
|
Part IV: Remarks regarding general conditions of the cargoes: (hygiene, package, label, humidity, etc.):
(Location and date)
|
Representative of deliverer |
Deliverer |
Recipient |
Representative of
recipient
|
Form:
VACCINE TRANSFER LOG
Vaccine delivery destination: …………………………
1/ Refrigerated truck – License plate: …………………….
2/ Date and time of transfer: ………………………………………
3/ Temperature at the time of dispatch: ………………………………………………
4/ Packaging conditions at the time of dispatch: ………………………………………………
5/ Transferring relevant vaccine documents:
|
- Notice of dispatch: |
Yes |
No |
|
- Record of vaccine transfer: |
Yes |
No |
|
- Goods-dispatch note: |
Yes |
No |
6/ Details on vaccine packaging:
|
No. |
Name of vaccine |
Number of containers/boxes |
Number of individual containers (if any) |
Number of doses |
Note |
|
1 |
|
|
|
|
|
|
2 |
|
|
|
|
|
|
3 |
|
|
|
|
|
|
4 |
|
|
|
|
|
|
5 |
|
|
|
|
|
|
6 |
|
|
|
|
|
|
7 |
|
|
|
|
|
|
8 |
|
|
|
|
|
|
9 |
|
|
|
|
|
|
10 |
|
|
|
|
|
|
11 |
|
|
|
|
|
|
12 |
|
|
|
|
|
|
13 |
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
Countersignature of driver/transporter
|
Vaccine storage keeper
|
Form
GOODS-DISPATCH NOTE
(Location and date)
Full name of recipient:
Reason of dispatch:
Dispatched commodities:
|
No. |
Name, label, and properties |
Unit |
Quantity |
Unit price |
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total: |
|
|
|
|
Total amount (in words):
(Location and date)
|
Superior |
Personnel in charge of distributing storage facility |
Dispatch note producer |
Recipient |
Storage keeper
|
GUIDELINES ON TRANSPORTING VACCINE WITH COLD BOXES
1. Objectives
These guidelines aim to provide instructions on how to transport vaccine using cold boxes in a manner that preserves vaccine in appropriate temperature.
2. Equipment and relevant documents
- Official Dispatch/Decision on distributing vaccine.
- Cold boxes, ice-packs, and thermometer.
- Means of transport: trucks, etc.
- Record of delivery, goods-dispatch note, certificate of vaccine dispatch.
- Information on recipient: Address, phone number of personnel in charge of receiving facility or superior in charge of receiving facility.
3. Procedures
Step 1: Producing vaccine transportation plan
Superior in charge of vaccine storage and personnel in charge of vaccine storage in military regions shall:
- Produce plans for distributing and transporting vaccine with specific quantity, type, batch of vaccine, etc. and estimated time of delivery to receiving facility. Notify receiving facility about transport plans (estimated time of vaccine delivery, number and type of vaccine).
- Dispatch automobiles and assign drivers as per prepared plan.
- Inform drivers:
+ Expected route, time, location of delivery, full name and phone number of personnel in charge at receiving facility.
+ Prior to delivery date, inspect technical conditions of vehicles such as tires, brake, gasoline, oil, machinery, refrigeration system.
Step 2: Packing vaccine and diluents in cold boxes
Storage personnel, vaccine storage assistant/transport personnel shall:
- Prepare vaccine transfer documents as per Procedure No. 12: Vaccine allocation and distribution
- Packing vaccine and diluents in cold boxes as per Procedure No. 7: Packing vaccine in cold boxes using ice-packs.
Step 3: Loading cold boxes onto automobiles and transporting
Storage personnel, vaccine storage assistant/transport personnel, and drivers shall:
- Immediately load cold boxes containing vaccine of each vaccine recipient onto automobiles and arrange the cold boxes in order, firmly in order to avoid collision and falling, and in a manner that allows easy distribution based on expected route (cold boxes to be distributed to the first receiving facility shall be placed in the outermost position).
- Lock the door leading to cargo compartments with care and immediately transport vaccine to receiving facility as per itinerary.
- Sign vaccine delivery log (Annex 2), storage personnel shall transfer relevant documents to transport personnel (goods-dispatch note, record of delivery, dispatch permit of each vaccine batch) and temperature monitoring instrument.
During transport process, transport personnel and drivers shall:
- Difficulties during transportation should be immediately reported to superior and personnel of distributing storage facility as per Procedure for responding to incidents during transport process.
- In case of vehicle malfunction where repair takes little time, repair vehicle engine in order to transport vaccine to receiving facility.
- In case repair takes a lot of time or cannot be performed, immediately contact the nearest receiving facility or the nearest military body in order to receive support as well as equipment and machinery in order to repair or dispatch other vehicle to continue the delivery.
- 45-60 minutes prior to arriving at vaccine delivery location, contact respective storage personnel to cooperate in receiving vaccine.
Step 4: Transferring vaccine
- Upon arrival, transport personnel shall immediately transfer vaccine and relevant documents (goods-dispatch note, record of delivery, dispatch permit) to storage personnel of receiving facility.
- Cooperate with receiving storage personnel in examining and noting time of delivery and temperature of cold boxes before transfer in record of delivery.
- Transfer adequate number of vaccine to receiving facility.
- Cooperate with storage personnel of receiving facility in inspecting quantity, batch, expiry date of vaccine and diluents delivered.
- Any discrepancy in terms of quantity, batch, or expiry date of vaccine/diluents found by storage personnel of receiving facility must be reported to storage personnel of military regions or superior of storage facility for solution.
- Fill in all information on time of transfer and conditions of vaccine in record of transfer. Collect signature and seal of superior of receiving facility.
- If any irregularity is found during transfer process, receiving storage facility has the right to reject the vaccine. In such case, receiving storage personnel must produce record and state reason for rejection, request their superior to confirm, and immediately inform superior of storage facility of military regions for timely solutions.
During the period awaiting solution, vaccine must be preserved separately in cold boxes or refrigerators as per preservation requirements.
- Retrieve cold boxes and temperature monitoring instrument, and return to distributing storage facility.
Step 5: Transferring cold boxes, thermometer, documents, and backing up data on vaccine temperature during transportation
- After returning to distributing storage facility, transport personnel are responsible for immediately transferring cold boxes, thermometer, record of delivery/goods-dispatch note of the delivery to storage personnel.
Storage personnel shall distribute and back up data on vaccine preservation temperature in cold boxes during vaccine delivery. If vaccine temperature reading during delivery process is not satisfactory to manufacturer’s requirements (i.e. situating outside of +2 oC to +8 oC), transport personnel and storage personnel must acknowledge the situation, report, and clarify the cause.
At the end of each delivery session, storage personnel shall submit reports to heads of military region.
Form:
SOCIALIST REPUBLIC
OF VIETNAM
Independence – Freedom – Happiness
----------------
RECORD OF VACCINE DELIVERY
Vaccine deliverer:
- Full name of representative: ………………………. Titles:
- Full name of deliverer: ………………………. Titles:
Vaccine recipient:
- Full name of representative: …………………………. Title:
- Full name of recipient: ………………………. Titles:
At ………, …………………… (time and date), the parties proceed to transfer vaccine as follows:
Part I: Documents on vaccine transfer:
|
|
Number of Official Dispatch on distribution of vaccine |
Date of signing Official Dispatch |
Permit of National Control Institute |
Goods-dispatch note |
|
Before receiving |
|
|
Yes □ No □ |
|
|
Upon receiving |
|
|
Yes □ No □ |
Yes □ No □ |
Part II: Details on received vaccine and diluents:
|
Name of vaccine |
Unit |
Quantity |
Number of batch |
Expiry date |
Manufacturer |
Manufacturing country |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluent |
Unit |
Quantity |
Number of batch |
Expiry date |
Manufacturer |
Manufacturing country |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Part III. Conditions of temperature monitoring instrument during transport
|
Total examined cargoes: |
Vaccine temperature: oC |
|||
|
Temperature monitoring instrument: |
VVM □ (stage 1, 2, 3, and 4) |
Freeze tags □ (write X or V) |
LogTag device Yes □ No □ |
|
Part IV: Remarks regarding general conditions of the cargoes: (hygiene, package, label, humidity, etc.):
(Location and date)
|
Representative of deliverer |
Deliverer |
Recipient |
Representative of
recipient
|
Form:
VACCINE TRANSFER LOG
Vaccine delivery destination: …………………………
1/ Refrigerated truck – License plate: …………………….
2/ Date and time of transfer: ………………………………………
3/ Temperature at the time of dispatch: ………………………………………………
4/ Packaging conditions at the time of dispatch: ………………………………………………
5/ Transferring relevant vaccine documents:
|
- Notice of dispatch: |
Yes |
No |
|
- Record of vaccine transfer: |
Yes |
No |
|
- Goods-dispatch note: |
Yes |
No |
6/ Details on vaccine packaging:
|
No. |
Name of vaccine |
Number of containers/boxes |
Number of individual containers (if any) |
Number of doses |
Note |
|
1 |
|
|
|
|
|
|
2 |
|
|
|
|
|
|
3 |
|
|
|
|
|
|
4 |
|
|
|
|
|
|
5 |
|
|
|
|
|
|
6 |
|
|
|
|
|
|
7 |
|
|
|
|
|
|
8 |
|
|
|
|
|
|
9 |
|
|
|
|
|
|
10 |
|
|
|
|
|
|
11 |
|
|
|
|
|
|
12 |
|
|
|
|
|
|
13 |
|
|
|
|
|
|
14 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
|
|
|
Countersignature of driver
|
Vaccine storage keeper
|
GOODS-DISPATCH NOTE
(Location and date)
No.
Full name of recipient:
Reason of dispatch:
Dispatched commodities:
|
No. |
Name, labels, and properties |
Unit |
Quantity |
Unit price |
Amount |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total: |
|
|
|
|
Total amount (in words):
(Location and date)
|
Superior |
Personnel in charge of distributing storage facility |
Dispatch note producer |
Recipient |
Storage keeper
|
GUIDELINES ON MAINTAINING TCW 4000AC REFRIGERATOR
1. Objectives:
These guidelines aim to provide instructions on how to perform daily and monthly maintenance for TCW 4000AC refrigerator in order to reduce possible risks that affect vaccine quality.
2. Equipment and relevant documents
- Required equipment: soft brush, clean cloth, and tray.
- Instruction manual of TCWC 4000AC refrigerator
- Operational diary.
- Cold boxes containing prepared ice-packs
- Refrigerators at required temperature for vaccine preservation in case of temporary transfer for maintenance.
3. Procedures
3.1. Daily tasks
- Examine refrigerator operation: examine connection to electrical grid and connection of temperature monitoring instrument.
- Clean dust collected on the outside of the refrigerator.
3.2. Weekly tasks
- Examine the drip pan, discard collected water, and examine any water collected in refrigerator compartment. Clear water drainage pipe in case of clogged drainage pipe.
- Examine airtightness of refrigerator washers and clean the washers.
- Examine frosting and defrost if frost is collected in the evaporator or on the refrigerator above 0.5 cm. Defrost as follows:
+ Step 1: Transfer vaccine and diluents to safe storage space: cold boxes containing ice-packs OR another refrigerator.
+ Step 2: Cut power source of the refrigerator and unplug the power plug.
+ Step 3: Open refrigerator door and wait until frost has completely melted. Do not use knife or other pointy object to pry the frost.
+ Step 4: Wipe the inside of the refrigerator with clean cloth.
+ Step 5: Examine shelves and equipment within the refrigerator.
+ Step 6: Close the refrigerator door.
+ Step 7: Turn on power source. Monitor refrigerator temperature until required temperature is achieved and maintained (usually after 24 hours).
+ Step 8: Transfer vaccine and diluents back in the refrigerator
3.3. Monthly tasks
a. Clean refrigerator cover or door with diluted soap water.
b. Clean a TCW 4000AC refrigerator as follows:
NOTE: Prior to cleaning a TCW 4000AC refrigerator, remove all vaccine from the refrigerator in order to temporarily store in a cold box containing ice-packs OR another functional refrigerator.
• Shut the power and unplug the power plug.
• Prepare temporary storage equipment and transfer vaccine from the refrigerator to the backup storage equipment kept at required temperature.
• Remove the plastic cover of the container and use a screw driver to remove the temperature recording piece on the inside of the compartment and the inside container.
• Use warm water and neutral cleaning solution to clean all equipment of the refrigerator, its cover ,and washers. Wipe dry with care.
• After cleaning the TCW 4000AC refrigerator, place inner container back to its position and install the temperature monitoring piece. Reattach the plastic cover of the container. Close the refrigerator door.
• Examine compressor and thermostat. The refrigerator would not function properly if these equipment collect dirt. Clean compressor and thermostat as follow:
+ Expose the side of the refrigerator that house the hot equipment and the compressor compartment.
+ Use a screw driver to unscrew the compressor and remove the protective cover.
+ Remove dust collected on the thermostat and in the compressor compartment with a soft brush.
+ Close the protective cover and fix the screw.
• Plug into power source and turn the power on.
• Operate the refrigerator and monitor temperature displayed on the monitor and automatic Fridge-tag 2.
• Only transfer vaccine from temporary storage back in the TCW 4000AC refrigerator once the refrigerator temperature is within +2 oC and +8 oC.
c. Storage personnel shall supervise implementation while maintenance personnel specify date and details of maintenance work in Operational diary, provide feedback on operation period and conditions of the refrigerator.
Operational diary sample
REFRIGERATOR
INFORMATION
AND OPERATIONAL DIARY
NAME OF REFRIGERATOR:.................................................................................
REFRIGERATOR INFORMATION
I. GENERAL INFORMATION
1. Manufacturer/Origin: ..................................................................................................
2. Refrigerator model, type: ……………………………………………………………………
3. Serial number: ………………………………………………………………………………..
4. Date of bringing into use: ……………………………………………………………………
II. TECHNICAL SPECIFICATIONS:
1. Voltage: …………………………………………………………………………..
2. Capacity: ………………………………………………………………………………………
3. Dimension: …………………………………………………………………………………….
4. Weight: ……………………………………………………………………………………
5. Other information: ……………………………………………………………………………….
Entity in charge:
III. INFORMATION ON SUPPLIER AND WARRANTY PROVIDER
1. Supplier:
Name of supplier:...........................................................................................
Address: ……………………………………………………………………………………………
Phone number: …………………………………………………………
2. Warranty provider:
Name of warranty provider: ...........................................................................................
Address: …………………………………………………………………………………………
Phone number: ……………………………………………………………………………………
IV. MANAGEMENT/REPAIR, REPLACEMENT/MAINTENANCE:
|
TIME |
INDIVIDUAL IN CHARGE |
WORK ENTRY |
NOTE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GUIDELINES FOR RESPONDING TO EMERGENCIES OF VACCINE PRESEREVED IN REFRIGERATORS
1. Objectives
These guidelines aim to instruct how to respond in case of emergencies (prolonged power outage, fire, natural disasters, flood, malfunction, etc.) in order to maintain safety of vaccine preserved in refrigerators and safety for humans.
2. Relevant equipment and tools
- Backup generators and fuel.
- Firefighting regulations posted in storage and firefighting facilities.
- Operational diary.
- List of name and phone number for contact in an emergency of storage keepers, specialized storage personnel, superiors of storage, and superiors of entities.
3. Procedures
Principles: In case of an emergency, maintain preservation temperature from 2 oC to 8 oC for all vaccine.
3.1. Scenario 1: PROLONGED POWER OUTAGE
In case of prolonged outage or case where specific time of power resupply is unknowable which may affect vaccine quality, proceed as follows:
|
No. |
Entry |
Assignment |
|
1 |
Immediately inform direct superiors. |
Individuals discovering the incident |
|
2 |
Operate backup generator(s) (if any) and guarantee sufficient fuel for the generator(s). If the entity does not have any backup generator, treat vaccine as per procedures applicable to malfunctioning refrigerator. Refrain from opening refrigerator door during the period of power outage |
Technician in charge |
|
3 |
Once backup power supply is restored, specify refrigerator temperature at the time of backup power restoration in operational diary. Cooperate with technicians in inspecting operational conditions of refrigerator. - In case of functional refrigerator: Continue to preserve vaccine as per regulations. - In case of non-functional refrigerator: Inform personnel directly in charge to rectify and remediate. |
Storage personnel/technicians |
|
4 |
Upon receiving grid power. Storage personnel must inspect, monitor operation of all refrigerators, and guarantee functional operation. Record refrigerator temperature (30 minutes after grid electricity has been provided and once every following 30 minutes for the first 2 hours to guarantee functional operations) and operating conditions of the refrigerator(s) in operational diary. |
Storage personnel/technicians |
3.2. Scenario 2: FIRE AND/OR EXPLOSION IN COLD STORAGE
When cold storage is met with fire, comply with following steps:
|
No. |
Entry |
Assignment |
|
1 |
Comply with Regulations on firefighting (alert, shutdown power source, call 114, employ available fire-fighting facilities, etc.) |
Individuals discovering the incident |
|
2 |
Move the refrigerator(s) to safety (if possible). Note: Do not open the refrigerator. |
All personnel |
|
3 |
Once firefighting is completed. - If the refrigerator(s) can operate functionally: Operate the refrigerator(s) as usual. List and examine vaccine and refrigerator temperature. - If the refrigerator(s) cannot operate functionally: + Transfer vaccine in damaged refrigerator(s) to other cold equipment and place in separate positions. + List all affected vaccine and report to the superiors. |
Storage personnel/technicians |
3.3. Scenario 3: TEMPERATURE FALLS OUT OF REGULATED RANGE DUE TO MALFUNCTIONAL REFRIGERATOR
Upon detecting refrigerator temperature falls out of safety range for preserving vaccine, comply with following steps:
|
No. |
Entry |
Assignment |
|
1 |
Record refrigerator at the time of discovery in operational diary. |
Storage personnel Specialized personnel of storage |
|
2 |
Immediately inform personnel in charge and technicians to rectify in a timely manner. |
Storage personnel Specialized personnel of storage Technicians |
|
3 |
Cooperative solutions: - If temperature drops below 2 oC: open the refrigerator door until temperature reaches 7 oC and close, then monitor closely. If suspecting vaccine is frozen, perform “shake test” for all vaccine susceptible to freezing. - If temperature surpasses 8 oC: Purchase ice and place in close nylon bags or trays, put in the refrigerator(s), and monitor temperature once every 30 minutes in order to maintain temperature within 2 oC and 8 oC - Transfer vaccine to another refrigerator or a cold box (if any) for separate preservation as per procedures for preserving vaccine in refrigerator and cold box. - In case of transferring vaccine to a cold box, vaccine must be packed as per procedures for packing vaccine into cold box - In case of transferring vaccine to another refrigerator, the refrigerator must be operating functionally at required temperature prior to transferring the vaccine |
Storage personnel Specialized personnel of storage |
|
4 |
Report to superiors for further solutions. |
Specialized personnel of storage |
3.4. Scenario 4: OTHER IRREGULARITIES (falling trees, flood, etc.)
- Step 1: Alert the entire facility
- Step 2: Examine and assess refrigeration system in order to provide rapid on-site remedial solutions:
○ If affected cold storage is facing risks of power outage, fire, explosion, or flood: Cut the power source that supplies the cold storage system
○ Employ available means to remediate the incident and cooperate in transporting vaccine from damaged refrigerator to safe location: E.g. transport vaccine from the first story to the second story (in case of flood) or cover the roof with flexible sheet (in case of blown off roof) or deal with unstable temperature as instructed under scenario 3.
GUIDELINES ON ALLOCATING AND DISTRIBUTING VACCINE
1. Objectives
These guidelines aim to provide instructions on how to allocate and distribute vaccine in the facility in a manner that guarantees sufficient amount of vaccine in a timely fashion.
2. Equipment and documents
- Plans on vaccine distribution, vaccine logbook, records of delivery/goods-dispatch note.
- Cold boxes, ice-packs (or ice), temperature monitoring instrument.
- Procedures for packing vaccine into cold boxes.
3. Procedures
3.1. Prepare cold boxes and ice-packs (or ice) in adequate amount and volume to preserve vaccine each time: Storage personnel or vaccine delivery/recipient shall implement.
3.2. Washing hands: Personnel must wash hands before holding vaccine containers and vials.
3.3. Vaccine delivery/receipt area must be able to avoid weather affect such as: direct sunlight, rain, dust, etc.
3.4. Distribute vaccine and/or diluents
|
No. |
Entry |
Assignment |
|
1 |
Inform distributed facility about time, quantity, and type of vaccine to enable distributed facility to prepare plans for receiving vaccine. |
Officials in charge of vaccine storage specialty |
|
2 |
Transfer Official Dispatch/Decision on distribution to accounting department and vaccine storage in order to produce vaccine dispatch and distribution note. |
Officials in charge of vaccine storage specialty |
|
3 |
Examine Official Dispatch/Decision on distributing vaccine and diluents regarding recipient, type of vaccine, quantity, batch number, expiry date. |
Vaccine storage personnel |
|
4 |
Record of vaccine delivery. |
Vaccine storage personnel |
|
5 |
Calculate number of cold boxes and ice-packs to prepare in advance Prepare cold boxes and ice-packs as per Procedures for packing vaccine into cold boxes. |
Vaccine storage personnel Assistant personnel |
|
6 |
Inspect temperature monitoring instrument of the refrigerator at the time of distribution. Record temperature data in dispatch note/record of delivery. |
Vaccine storage personnel Vaccine delivering personnel |
|
7 |
Collect necessary vaccine by type, quantity, batch number from the refrigerator. Cross check, deliver/receive each type of vaccine based on dispatch note, and pay attention to number of batch and expiry date of vaccine Arrange and pack vaccine into cold boxes as per Procedures for packing vaccine into cold boxes. Vaccine and diluents shall be packed in cold boxes of their corresponding manufacturers. Specify type of packaging. |
Vaccine storage personnel Assistant personnel |
|
8 |
Sign transfer log, record of delivery/goods-dispatch note. |
Vaccine storage personnel Vaccine delivering personnel |
|
9 |
Record distributed facility, distributed amount, temperature, and time of distribution in vaccine logbook |
Vaccine storage personnel |
|
10 |
Store Official Dispatch/Decision on distribution, dispatch note, and record of vaccine delivery in storage. |
Vaccine storage personnel |
GUIDELINES ON EXAMINING VACCINE AND DILUENTS
1. Objectives
Examination of vaccine and diluents aims to accurately identify quantity, batch, expiry date, and current conditions of each type of vaccine and diluents in store in order to allow a more effective management and distribution of vaccine, guarantee sufficient vaccine, and limit expiration of vaccine. These guidelines provide steps for examining vaccine and diluent storage on a quarterly basis or after every vaccine receipt/supply.
2. Equipment and relevant documents
- Decision on establishment of Council for examination (if necessary/in inspection and examination session).
- Note of receipt/dispatch of vaccine, diluents.
- Record of receipt/transfer of vaccine, diluents.
- Vaccine accounting book (if any).
- Vaccine, diluent log.
- Examination record of the previous examination.
3. Procedures
3.1. Examination after each vaccine receipt/supply
|
No. |
Entry |
Assignment |
|
1 |
Prepare: vaccine and diluent log, note of receipt/dispatch of vaccine, vaccine receipt/transfer record |
Vaccine storage personnel |
|
2 |
Examine at vaccine storage |
Officials in charge of vaccine storage specialty Vaccine storage personnel Vaccine storage assistants |
|
2.1 |
Examine each type of vaccine and diluent: + Quantity. + Batch number. + Expiry date. + Conditions of labels of vaccine vials, color, and consistency of vaccine, diluents. + Indicators on vaccine labels (if any) |
|
|
2.2 |
Cross check between vaccine, diluent log with actual quantity in store |
|
|
4 |
Record post-examination figures in vaccine, diluent log Any discrepancy requires identification of cause and appropriate solutions. |
Officials in charge of vaccine storage specialty Vaccine storage personnel |
3.2. Quarterly examination:
|
No. |
Entry |
Assignment |
|
1 |
Inform examination plans, and prepare record of examination. |
Officials in charge of vaccine storage specialty Vaccine storage personnel |
|
2 |
Prepare: vaccine and diluent log, note of receipt/dispatch of vaccine, vaccine receipt/transfer record, inspection record of the previous inspection. |
Vaccine storage personnel |
|
3 |
Examine at vaccine storage |
Superior of officials in charge of vaccine storage specialty Vaccine storage personnel Relevant personnel Vaccine storage assistants |
|
3.1 |
Examine each type of vaccine and diluent: + Quantity. + Batch number. + Expiry date. + Conditions of labels of vaccine vials, color, and consistency of vaccine, diluents. |
|
|
3.2 |
Cross check between vaccine, diluent log with actual quantity in store |
|
|
4 |
Complete examination record. Any discrepancy requires identification of cause and appropriate solutions. |
Officials in charge of vaccine storage specialty Vaccine storage personnel |
|
Submit examination report to superior and store in department. |
Officials in charge of vaccine storage specialty Vaccine storage personnel |
GUIDELINES ON RETRIEVING AND STORING VACCINE IN ISOLATION
1. Objectives
These guidelines aim to provide steps for retrieving and storing vaccine in isolation for vaccine suspected in terms of quality or vaccine awaiting disposal to ensure adequate retrieval, avoid using such vaccine batches, and ensure safe, effective vaccine use.
2. Equipment and relevant documents
- Decisions/documents of Ministry of Health informing and guiding temporary cessation of use or retrieval of a type or a batch of vaccine.
3. Procedures
- Designate an area/position in the storage to store vaccine in isolation awaiting disposal: install locks or physical separation for unqualified vaccine.
- Designate a refrigerator or several sections in a refrigerator to store vaccine awaiting resolution (awaiting inspection/direction from superior)
- In case of storage in the same refrigerator with other vaccine, pack and seal separately, install physical separation with distinctive marking to avoid confusion
|
No. |
Entry |
Assignment |
|
1. |
After receiving documents notifying retrieval/temporary cessation of use of vaccine, receiving entity must examine and submit detail notice on type of retrieved/ceased vaccine (batch number, expiry date, preservation/transport solutions (preservation in cold temperature or regular temperature)) and deadline for retrieval to relevant entities. |
Entity in charge of retrieving Relevant entity |
|
2. |
List quantity of vaccine/diluents to be suspended or retrieved of each entity and report to superior |
Specialized personnel of storage Vaccine storage personnel |
|
3. |
Prepare a separate storage area in the entity or separate refrigerator with appropriate cold storage volume to store vaccine suspended/retrieved and stored in isolation awaiting resolution In case separate refrigerator is not available, prepare a separate section in a refrigerator with physical separation and distinctive marking to store vaccine which must be packed, sealed, and stored in isolation to avoid confusion. |
Vaccine storage personnel |
|
4 |
Retrieve vaccine: in case of retrieving vaccine from entities in order to preserve in storage of military regions, transfer/receive vaccine as per Procedures No. 9 and Procedures No. 1. |
Vaccine storage personnel Vaccine transfer/receipt personnel Specialized personnel of storage |
|
5. |
Store vaccine in isolation: - In case suspended vaccine must be stored in isolation awaiting disposal: + Preserve vaccine in refrigerator or separate sections in a refrigerator as per Procedures No. 2 until disposal measures are provided. + Attach a yellow sign with black letters “Vắc xin biệt trữ chờ xử lý” (Vaccine stored in isolation awaiting disposal) on the refrigerator or containers of said vaccine. + For distributed vaccine that must be returned to storage due to incidents during transportation, after inspection and confirmation have been performed and removal from cold boxes and/or refrigerated trucks has been done, assign separate sections in refrigerator, monitor quality and prioritize this vaccine for distribution - For vaccine stored in isolation awaiting disposal: + Bring vaccine awaiting disposal out of cold chain and into isolation storage awaiting disposal in storage with red sign and black letters that reads “Vắc xin biệt trữ chờ hủy” (Vaccine stored in isolation awaiting disposal). + Pack and seal vaccine containers, specify information such as name of vaccine, quantity, batch number, and expiry date on containers. + Adopt procedures for disposing vaccine as per procedure to ensure safety for humans and the environment. |
Vaccine storage management personnel. Specialized personnel |
|
6. |
Adopt procedures for disposing vaccine as per procedure to ensure safety for humans and the environment. Submit report on retrieval, storage in isolation/disposal of vaccine to heads of Military Region and Steering Committee of the campaign. |
Specialized personnel Head of entity |
GUIDELINES ON RESPONDING TO INCIDIENTS DURING TRANSPORTATION
1. Objectives
These guidelines provide instructions on how to respond to incidents during transportation of vaccine with refrigerated trucks in order to minimize adverse effect during transportation that effects vaccine quality; maintain preservation temperature of +2 °C to +8 °C during transportation period.
2. Equipment and relevant documents
- Mobile phone
- List of recipients on transport route
- Procedures for responding to incidents in preservation vaccine in refrigerators.
- Backup costs for purchasing fuel and repairing vehicles
3. Steps of implementation
|
No. |
Entry |
Assignment |
|
4.1. Incidents that occur on the road |
|
|
|
1 |
Call superior/specialized personnel of storage to inform about the situation and request solutions |
Driver/vaccine transporter |
|
2 |
a. For refrigerated trucks: Immediately adopt necessary measures depending on situations below: * In case of engine malfunction: - Refrigerated trucks are located near a power source: Operate refrigeration system with said power source. - Refrigerated trucks are not located near any power source or cannot operate on a power source: If temperature of refrigerated compartment exceeds 8 oC, purchase ice along the way to maintain preservation temperature. Do not use dry ice which can cause preservation temperature to drop below 0 oC and cause poisoning. - In case vehicle repair takes little time, repair vehicle engine in order to transport vaccine to receiving facility. - In case vehicle repair takes too much time or cannot be done, drivers must contact the nearest entities for support. * In case of refrigeration malfunction - If temperature of refrigeration compartment exceeds 10 oC, purchase ice along the way to maintain preservation temperature. - If refrigerated trucks are located near the storage, drivers must immediately return to the storage and inform superior of vaccine storage for plans for receiving vaccine. - In case refrigerated trucks are not located near the storage + Contact the nearest entities for support and send vaccine to local storage or request refrigerated trucks of local governments for vaccine transport. In case of sending vaccine to local storage, drivers must transfer the vaccine to storage keepers and request storage keepers to preserve vaccine as per required temperature. + Contact superiors to dispatch vehicles from the Military Region to continue the transport. - When transferring vaccine to another refrigerated truck, drivers must also transfer the temperature monitoring instrument that comes with the vaccine. - Repair refrigeration system to continue to transport vaccine to receiving facility (if possible). During period of incident, transporters/drivers must frequently contact specialized personnel of storage for recommendations. * In case of malfunctioning of other components: If refrigerated compartment is functional, repair in order to transport vaccine to receiving facility. |
Driver/vaccine transporter Storage personnel Specialized personnel of storage Head of entity |
|
3. |
b. For regular trucks: Adopt necessary measures in case of engine malfunction as follows: - In case vehicle repair takes little time, repair vehicle engine in order to transport vaccine to receiving facility. - In case repair takes too much time or cannot be done: + Contact the nearest provincial entities for assistance and assignment of local vehicles for transport or contact heads of storage for assigning vehicles of the Military Region to assist. + If preservation temperature in refrigerated compartment exceeds 10 oC, purchase ice along the way to maintain temperature within +2 °C and +8 °C (contain ice in nylon bags to prevent water from peeling the vaccine label off) |
Driver/vaccine transporter Storage personnel Specialized personnel of storage Head of entity |
|
4.2. Upon returning to storage |
||
|
1 |
Driver/transporter must immediately submit detail report on the situation to their superior/specialized personnel while transferring vaccine delivery record and temperature monitoring instrument to vaccine storage management personnel. In case vaccine is returned to storage, classify and deal with vaccine as per Procedures No. 11 and Procedures No. 14. |
Driver/vaccine transporter Storage personnel Specialized personnel of storage Head of entity |
|
2 |
Vaccine storage management personnel shall read the temperature monitoring instrument, review the documents, and submit reports to heads of entities/Military Region for presentation to Steering Committee of the campaign |
Specialized personnel of storage Head of Military Region |
List of contact in case of emergency (name/phone number):
+ Head of storage: ………………………………………………..
+ Specialized personnel of storage: ……………………….
+ Storage personnel: ………………………………
GUIDELINES ON TRANSPORTING COVID-19 VACCINE FROM AIRPORTS TO STORAGE FACILITIES
1. Objectives
The COVID-19 vaccines licensed for use in Vietnam have 3 recommended temperature ranges: from 2 oC to 8 oC, from –25 oC to –15 oC, and negative/ultra-low temperature. Time for preservation of each type of vaccine in each temperature range varies. Vaccines recommended for preservation in ultra-low temperature after thawing are generally usable within up to 30-31 days. Thus, transportation of vaccine should be prioritized and promoted to ensure vaccination safety and effectiveness.
In order to ensure effectiveness in vaccine transportation and preservation as well as maximize preservation capacity, and the fastest delivery time to vaccination facilities, procedures for transporting COVID-19 vaccine must be complied under close cooperation between entities affiliated to Ministry of National Defense and entities affiliated to Ministry of Health in the COVID-19 vaccination campaign in 2021-2022.
2. Procedures
2.1. Vaccine to be preserved within 2 oC to 8 oC:
|
|
|
|
|
|
|
|
|
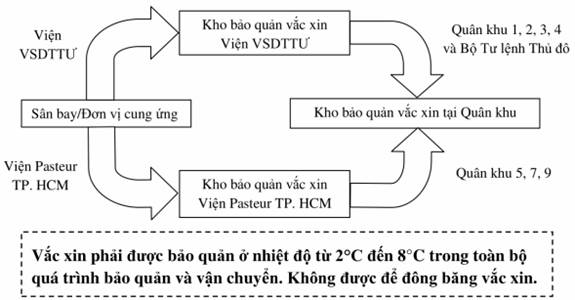
- The National Institute of Hygiene and Epidemiology (NIHE) shall complete procedures for importing COVID-19 vaccine to be preserved within 2 oC to 8 oC from overseas, sponsors, or distributors in Vietnam. After which point, vaccine will be transported to vaccine storage facility in the NIHE and Pasteur Institute of Ho Chi Minh City.
- Once notice of release is issued and decision on vaccine distribution of Ministry of Health reaches military regions and provinces, the NIHE shall inform the General Department of Logistics, Department of Military Medicine, and Military regions affiliated to Ministry of National Defense about receiving and transporting vaccine, including: contact point, time, type of vaccine, number of doses, number of containers, etc.
- Based on information above, the General Department of Logistics must direct relevant entities to develop plans for receiving and transporting vaccine using refrigerated trucks maintained at a temperature from 2 oC to 8 oC from vaccine storage facility at the NIHE and Pasteur Institute of Ho Chi Minh City to storage facilities of Military regions.
- After receiving vaccine, officials in storage facilities of Military regions shall unload, categorize into batches, examine, and include in reports on receipt: type of vaccine, number of batches, quantity, expiry date, conditions upon receipt, etc. and submit to the NIHE (Annex 1).
- Vaccine shall be preserved at a temperature from 2 oC to 8 oC in specialized refrigerators in storage facilities of Military regions.
2.2. Vaccines preserved in negative/ultra-low temperature and eligible for preservation at 2 oC to 8 oC:
|
|
|
|
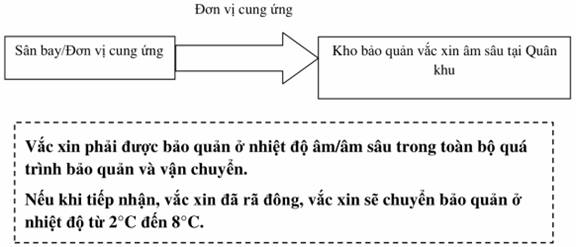
- Once notice on receiving COVID-19 vaccine to be preserved in negative/ultra-low temperature from overseas, sponsors, or distributors in Vietnam is received, Ministry of Health shall issue decision on distributing vaccine.
- Once the NIHE has completed procedures for receiving vaccine, based on decisions on vaccine distribution of Ministry of Health, distributors shall transport vaccine at negative/ultra-low temperature and transfer to storage facilities in Military regions.
- Officials in storage facilities of Military regions shall unload, categorize into batches, examine, and include in reports on receipt: type of vaccine, number of batches, quantity, expiry date, conditions upon receipt, etc. and submit to the NIHE after receiving (Annex 1).
- Follow the steps for returning containers to manufacturers (Annex 2).
- Vaccine shall be preserved in ultra-low freezers in storage facilities in Military regions. Apply the label that reads “Vắc xin chờ kiểm định, chưa được cấp phát” (Vaccine awaiting examination. Do not distribute).
- Collect specimen for examination: In case multiple vaccine batches are different from vaccine batches in preservation at the NIHE/Pasteur Institute:
+ After receiving report on receiving vaccine from Military regions, the NIHE shall immediately issue notice on number of vaccine doses to be collected from each batch for examination.
+ Military regions are responsible for transporting the specimen to the NIHE, the Pasteur Institute of Ho Chi Minh City, or National Institute for Control of vaccine and biologicals within 2 days after receiving the notice. Vaccine specimen collected for examination must be preserved at 2 oC to 8 oC during transportation.
- Once notice of dispatch is issued, Military regions are then allowed to distribute vaccine.
2.3. Vaccine to be preserved within -25 oC to -15 oC:
|
|
|
|
|
|
|
|
|
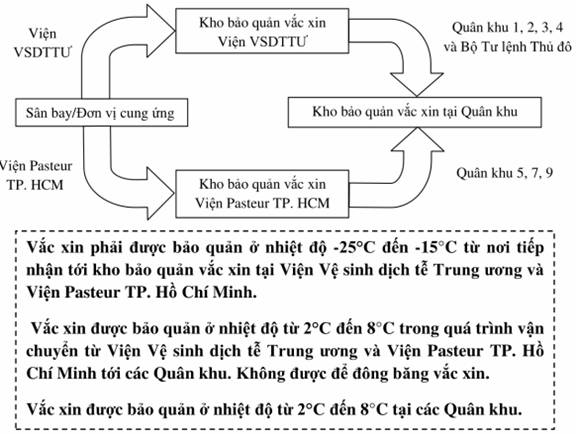
- The National Institute of Hygiene and Epidemiology (NIHE) shall complete procedures for importing COVID-19 vaccine to be preserved within -25 oC to -15 oC from overseas, sponsors, or distributors in Vietnam. After which point, vaccine will be transported to vaccine storage facility in the NIHE and Pasteur Institute of Ho Chi Minh City for preservation at a temperature from -25 oC to -15 oC.
- Once notice on dispatch is issued, Ministry of Health shall issue decisions on vaccine distribution.
- The NIHE shall inform the General Department of Logistics, Department of Military Medicine, and Military regions affiliated to Ministry of National Defense about receiving and transporting vaccine, including: contact point, time, type of vaccine, number of doses, number of batches, etc.
- Based on information above, the General Department of Logistics must direct relevant entities to develop plans for receiving and transporting vaccine using refrigerated trucks maintained at a temperature from 2 oC to 8 oC from vaccine storage facility at the NIHE and Pasteur Institute of Ho Chi Minh City to storage facilities of Military regions.
After receiving vaccine, officials in storage facilities of Military regions shall unload, categorize into batches, examine, and include in reports on receipt: type of vaccine, number of batches, quantity, expiry date, conditions upon receipt, etc. and submit to the NIHE (Annex 1).
Vaccine shall be preserved at a temperature from 2 oC to 8 oC in specialized refrigerators in storage facilities of Military regions.
GUIDELINES ON RETURNING VACCINE CONTAINERS TO MANUFACTURERS
Step 1: Receiving vaccine containers from suppliers
- Transporting DHL unit: Lift cardboard cover of vaccine containers, collect the purple instruction manual and printed return label on the top side of the container cover. Place the printed return label in nylon bags (transparent nylon bags that come with adhesives) provided by the DHL Company (return labels must come with the correct containers) together with invoices printed from available files.

Turn of temperature monitoring instrument Controlant logger by holding the button Stop shipment for 5 seconds, the device will blink 4 times before shutting down, at which point, release the button.

Make sure that temperature monitoring instrument Controlant logger is placed back into the containers in the original positions.

Step 2: Remove all dry ice from the containers after removing vaccine from the containers
Use white labels to cover the dry ice marker UN1845 & the 9-shaped rhombus as the containers no longer contain dry ice.

Note: All hazardous commodity labels (including those of transporting units) must be covered.
DO NOT COVER the lithium UN3481 battery label in red outline.
Step 3: Apply seal to cover the dry ice markings
Seals are taken from the backside of the purple instruction manual that comes with each container.
Note: DO NOT COVER the lithium UN3481 battery label in red outline.

Step 4: Apply nylon bags containing printed labels and invoices to the top left corner of a container (apply over the label covering the dry ice note)
Step 5: Seal container cover with transparent duct tape. Containers are then ready to be returned to manufacturers.










