Nội dung toàn văn Decision 5772/QD-BYT 2021 sample issuance of Vaccine passport
|
MINISTRY OF HEALTH |
SOCIALIST REPUBLIC OF VIETNAM |
|
No. 5772/QD-BYT |
Hanoi, December 20, 2021 |
DECISION
SAMPLE AND PROCEDURES FOR ISSUANCE OF “VACCINE PASSPORT”
MINISTER OF HEALTH
Pursuant to Law on Prevention and Control of Infectious Diseases in 2007;
Pursuant to Decree No. 75/2017/ND-CP dated June 20, 2017 of Government on functions, tasks, powers, and organizational structure of Ministry of Health;
At request of Director General of Agency of Information and Technology,
HEREBY DECIDES:
Article 1. Sample and procedures for issuance of “Vaccine passport” are attached hereto.
Article 2. Sample and procedures for issuance of “Vaccine passport” attached hereto shall be applied consistently in all vaccination centers across the country.
Article 3. This Decision comes into effect from the day of signing.
Article 4. Director General of Agency of Information and Technology, Director General of General Department of Preventive Medicine, Chief of Ministry Office, Chief of Minister Inspectorate, Directors and General Directors of entities affiliated to Ministry of Health; Directors of Departments of Health of provinces and central-affiliated cities; heads of medical sector and relevant entities are responsible for implementation of this Decision./.
|
|
PP. MINISTER |
SAMPLE AND PROCEDURES FOR ISSUANCE OF “VACCINE PASSPORT”
(Attached to Decision No. 5772/QD-BYT dated December 20, 2021 of Minister of Health)
1. Sample on “Vaccine passport”
1.1. Displayed fields of information:
1. Full name;
2. Date of birth;
3. Nationality;
4. Targeted disease;
5. Number of dose;
6. Date of vaccination;
7. Dose number;
8. Vaccine;
9. Vaccine product;
10. Vaccine supplier or manufacturer;
11. Certificate number.
The aforementioned information will be digitally signed and packed in form of QR code with 2D format. Specific requirements will be specified under Section 4.
1.2. The aforementioned information includes full name and date of birth together with other identification documents namely: ID Card, Citizen Identity Card, or Passport of the Vaccine passport holder.
1.3. Information on the target disease of the certificate, vaccine, type of vaccine and the manufacturer or supplier shall be displayed in accordance with the “COVID-19 vaccine tracker and landscape” of World Health Organizations (WHO) updated on WHO’s website and “Value Sets for EU Digital COVID Certificates” issued by the European Union (EU).
1.4. Vaccination date and number of doses received serve to determine vaccination information.
1.5. A QR code expires after 12 months from the date on which it is generated.

Figure 1: Illustration of electronic Certificate of COVID-19 vaccination of Vietnam on mobile device
2. Procedures for issuance of “Vaccine passport”
2.1. Procedure flowcharts
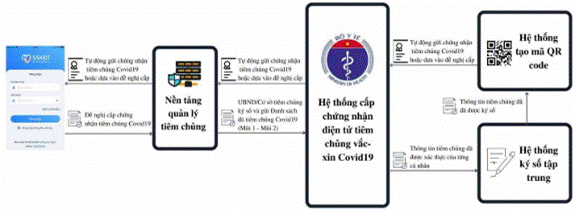
2.2. Description
- Step 1: Vaccination facilities shall review, verify, and confirm information of COVID-19 vaccine recipients in accordance with Official Dispatch No. 8938/BYT-DP dated October 21, 2021 of the Ministry of Health and Official Dispatch No. 9438/BYT-CNTT dated November 5, 2021.
- Step 2: Vaccination facilities shall
digitally sign COVID-19 vaccination data on the COVID-19 vaccination management
platform. The COVID-19 vaccination management platform shall connect and share
vaccination data with Certificate of COVID-19 vaccination management and
issuance system satisfactory to regulations on connection of medical data of
the Ministry of Health.
COVID-19 vaccination data on the COVID-19 vaccination management platform must satisfy requirements under Section 3 and Section 4.
- Step 3: General Department of Preventive Medicine - Ministry of Health shall digitally sign certificate of COVID-19 vaccination. The issued certificates shall use QR format under EU standards under Section 5.
National epidemic control applications and other applications (with the user’s consent) shall receive and store COVID-19 vaccination confirmation in form of QR code in accordance with guidance on exchanging medical data regulated by the Ministry of Health.
3. Standardization of COVID-19 vaccination data
|
No. |
Information |
Example |
|
|
Personal information |
|
|
1 |
Full name |
Nguyen Van A |
|
2 |
Date of birth |
20/10/1999 |
|
3 |
Phone number |
0912345678 |
|
4 |
ID Card/Citizen Identity Card number |
001123456789 |
|
5 |
Passport number |
If any |
|
6 |
Nationality |
Vietnamese |
|
|
The first dose |
|
|
7 |
Vaccine* |
EU/1/20/1528 |
|
8 |
Type of vaccine* |
1119305005 |
|
9 |
Manufacturer/supplier* |
ORG-100030215 |
|
10 |
Dose number |
1 |
|
11 |
Date of vaccination |
20/6/2021 |
|
12 |
Vaccinating entity |
|
|
13 |
Superior entity |
|
|
|
The second dose |
|
|
14 |
Vaccine * |
EU/1/20/1528 |
|
15 |
Type of vaccine * |
1119305005 |
|
16 |
Manufacturer/supplier * |
ORG-100030215 |
|
17 |
Dose number |
2 |
|
18 |
Date of vaccination |
20/7/2021 |
|
19 |
Vaccinating entity |
|
|
20 |
Superior entity |
|
|
21 |
Total doses received |
2 |
|
Digital signature of vaccination facility |
||
*Specify vaccine, type of vaccine, and manufacturer or supplier in accordance with Section 4. List of international code
4. List of international code
4.1. Epidemic
|
Code |
Name |
|
840539006 |
COVID-19 |
4.2. Type of vaccine
|
Code |
Name |
|
1119305005 |
SARS-CoV-2 antigen vaccine |
|
1119349007 |
SARS-CoV-2 mRNA vaccine |
|
J07BX03 |
Other Covid-19 vaccine |
4.3. Vaccine product
|
Code |
Name |
|
EU/1/20/1528 |
Comirnaty/ Pfizer |
|
EU/1/20/1507 |
Spikevax (previously COVID-19 Vaccine Moderna)/Moderna |
|
EU/1/21/1529 |
Vaxzevria/ Astrazeneca |
|
EU/1/20/1525 |
COVID-19 Vaccine Janssen/ Janssen |
|
Hayat-Vax |
Hayat-Vax/ Hayat-Vax |
|
BBIBP-CorV |
BBIBP-CorV/ Vero-Cell |
|
Sputnik-V |
Sputnik-V/ Sputnik |
|
Abdala |
Abdala/ Abdala |
4.4. Name of manufacturer/brand
|
Code |
Name |
|
ORG-100030215 |
BioNTech Manufacturing GmbH |
|
ORG-100031184 |
Rovi Pharma Industrial Services, S.A, Spain Recipharm Monts, France |
|
ORG-100001699 |
AstraZeneca, AB |
|
ORG-100001417 |
Janssen-Cilag International NV |
|
ORG-100023050 |
Gulf Pharmaceutical Industries |
|
ORG-100020693 |
Beijing Institute of Biological Products Co., Ltd |
|
CIGB |
Cuba CIGB (Center for Genetic Engineering and Biotechnology) |
5. Technical procedures for generating QR code
5.1. Procedures for generating QR code
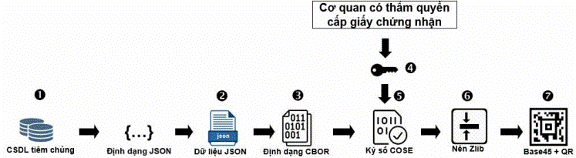
Figure 2: Procedures for generating QR code
5.2. Technical requirements for steps of QR code generation
|
No. |
Details |
Technical requirements |
|
1 |
Data format under section II |
JSON format (according to RFC 7159) |
|
2 |
Convert JSON data into CBOR |
Use algorithm to convert JSON format into CBOR format (according to RFC 8392) |
|
3 |
Digital signature |
Use 2084-bit RSA digital signature algorithm (according to RFC 8230) and SHA-256 hashing algorithm (according to ISO/IEC 10118- 3:2004) |
|
4 |
Encoding and compressing CBOR data |
Use COSE protocol (according to RFC 8152) |
|
5 |
Compressing data that has undergone COSE |
Use Zlib protocol (according to RFC1950) |
|
6 |
Altering data structure |
Use BASE45 algorithm |
|
7 |
Generate 2D image |
Use ASCII algorithm (according to part 3 of the ICAO 9303) and create QR barcode (according to ISO/IEC 18004:2015) |