Nội dung toàn văn Decision No. 407-QD science and technology, promulgating two standards
|
PRESIDENT OF THE COMMITTEE OF
SCIENCE AND TECHNOLOGY |
SOCIALIST REPUBLIC OF VIETNAM |
|
No. 407-QD |
Hanoi, November 20th 1984 |
DECISION
No. 407-QD - ISSUED BY THE COMMITTEE OF SCIENCE AND TECHNOLOGY, PROMULGATING TWO STANDARDS
THE PRESIDENT OF THE COMMITTEE OF SCIENCE AND TECHNOLOGY
Pursuant to the Decree No. 141-HĐBT dated August 24th 1982 of the Minister Council, issuing the Regulation on standardization;
At the request of the Ministry of Food Industry in the Official Dispatch No. 194-CNTP/KT dated October 24th 1983.
DECISION
Article – Promulgating two Vietnam’s Standards:
TCVN 3973-84. Edible salt (sodium chloride). Test methods.
TCVN 3974-84. Edible salt (sodium chloride). Technical requirements.
Article 2 – These standards come into effect from January 01st 1986, and shall be observed.
|
|
Doan Phuong (Signed) |
TCVN 3973-84.
EDIBLE SALT (SODIUM CHLORIDE) TEST METHODS
- Compiler: The Department of Salt Industry – The Ministry of Food Industry
- Requester: The Ministry of Food Industry
- Submitter: the Directorate for Standards, Metrology and Quality
- Issuer: The Committee of Science and Technology
- Promulgated together with the Decision No. 107/QĐ dated November 20th 1984.
This Standard specifies the method of sampling and determinating organoleptic, chemical, and physical indicators of edible salt (Sodium Chloride – NaCl).
1. Sampling.
1.1. The quality of a shipment of salt is determined based on the analysis of the average sample of the shipment.
A shipment of salt is an amount of salt in the same category, produced from the same technological line, and delivered at the same time.
The average sample is taken from the initial samples of the shipment. Before taking an initial sample, the form, impurities, and packaging conditions should be observed.
1.2. Taking initial samples.
1.2.1. Group all initial samples of the shipment into a general sample. The amount of initial samples must ensure that the general samples weigh at least 20 kg.
1.2.2. Salt consigned in piles, salt in warehouses, and salt on train cars.
Initial samples are taken from at least 03 trenches, 0.5 m deep, 0.5 m wide. Put a shovel to deep into the salt (do not scratch horizontally) at 9 points evenly distributed along the trench.
For big shipments of at least 1,000 tonnes, take a sample from every 20 tonnes evenly distributed along the height and the cross-section of the shipment. Samples shall be taken during the unloading of salt.
1.2.3. For salt in boats or barges.
Initial samples are taken 3 times while unloading: at the beginning of the unloading, after one fourth of the shipment is unloaded, and after three fourth of the shipment is unloaded. 10 samples shall be taken at various positions of the boat or barge.
For boats of 3-5 tonnes, 3 samples shall be taken from each boat.
1.2.4. For big bags or small bunches of salt.
Initial samples shall be taken from 3% of the bags or bunches. At least 3 bags or bunches shall be sampled. Samples shall be taken from sequential segments. For example: a shipment has 100 bags, the 20th, 50th, and 80th etc. shall be sampled.
1.3. Taking average samples.
Mix the general samples, evenly spread them over a clean plane to form a square. Divide the square into 4 triangles by its two diagonals, remove 2 opposite parts, and mix the remaining 2 parts. Repeat this process until 1.5 kg of sample remains. This is called an average sample.
Divide the average sample into 3 equal parts and put them into capped jars or 2-layer polyethylene bags. 1 sample shall be sent to a laboratory for analysis, 1 sample shall be retained for arbitration analysis, 1 sample shall be kept by the consignor or consignee.
The label on the average sample must indicate the producer, product name, quality class, shipment weight, sampling date and place, name of the sample taker.
The arbitration analysis agency and the place where the arbitration sample are selected by the consignor and consignee.
Notes:
1. The general sample is usually divided into 1.5-kg parts (average sample) at the sampling place before being taken to the laboratory.
2. Where salt is unloaded by conveyor belts or chutes, initial samples shall be taken when salt leaves the end of conveyor belt or chute. Use a 0.6 x 0.6 x 0.05 m tray to catch salt from its flowing stream. The weight of sample is similar to above.
1.4. Sampling tools.
All sampling tools must be dry and clean. Sampling tools and sample containers include: small knives, 50×50×20cm glaze or plastic trays (or 80×80×20cm wooden trays with plastic lining), wooden shovels (similar to a sand shovel), salt shovels, capped jars, 20 x 55 cm 2-layer polyethylene, tools for seaming and sealing bags, 2-layer jute or sedge bags (the inner layer is plastic) to contain initial salt samples.
2. Test methods.
In all tests, pure reagents shall be used for analysis.
2.1. Determination of color: evenly spread the sample on a white and clean plane until the salt is 1 cm thick; observe the color.
2.2. Determination of taste: Dissolve 5 g of salt in 100 ml of distilled water at ambient temperature; taste it to determine the taste.
2.2. Determination of smell: Grind 20 g of salt in a ceramic mortar at ambient temperature; smell it to determine the smell.
2.4. Determination of humidity.
2.4.1. Use a capped ceramic bowl and a heater that could produce a temperature of 140 oC.
2.4.2. Testing: heat the capped ceramic bowl at 140 oC and cool it down in a desiccator. Weigh the bowl, and keep heating it until the weight remains unchanged.
Put 10 g of salt (± 0.01 g) in the bowl. Heat the salt at 140 oC ± 10 oC for 3 hours. Cap the bowl, cool it in a desiccator, and weigh it. Keep heating it for 30 minutes and weigh it until the difference between two weighing times does not exceed 0.01 g.
2.4.3. Result calculation. Salt humidity is expressed as percentage (X1) using the formula below:
![]()
m1: weight of the bowl and salt before heating, expressed as gram; m2: weight of the bowl and salt after heating, expressed as gram.
M: weight of salt, expresses in gram.
2.5. Determination of water-insoluble impurity content.
2.5.1. Tools.
- Analysis scale, accuracy of 0.001g.
- Desiccator.
- Filter paper.
2.5.2. Testing process
Put 25 g of dried salt (salt dried until its weight remains unchanged) in a 400-ml beaker and dissolve it with 100 ml of distilled water.
Boil the solution for 10 minutes, cap the glass and keep boiling for 30 minutes; cool it down to ambient temperature. Pass the solution through a funnel with filter paper (the filter is dried and its weight is known). Rinsing and washing water is brought to the filter funnel. Rinse insoluble substances right on the filter paper many times by distilled water until no white precipitate of silver chloride is seen when making the washing water react silver nitrate 0.1N. Put the solution in a 500-ml volumetric flask; cool it down and add more distilled water to reach the mark; shake the cylinder to analyze ions.
The filter paper and insoluble substances shall be put on a glass dial, dried at 105 ± 2 oC for 3 hours, cooled down in a desiccator, and weighed. This process shall be repeated until the difference between two weighing times does not exceed 0.001 g.
2.5.3. Result calculation.
The content of impurities insoluble in water is expressed as percentage of dry matter (X2) using the following formula:
![]()
m1: Weight of the filter paper and heated insoluble substances, expressed in gram.
m2: Weight of the filter paper, expressed in gram.
m: Weight of salt, expressed in gram.
The result is the arithmetic mean of 2 calculations.
2.6. Determination of content of chlorine ions (Cl-)
2.6.1. Principles: Titrate chlorine ion by silver nitrate solution (AgNO3) with the potassium chromate (K2CrO4) as an indicator.
2.6.2. Reagents and tools.
- 0.1N silver nitrate solution.
- Potassium chromate, 10% solution.
- 250-ml volumetric flask.
2.6.3. Testing process: take 25 ml of salt solution from the 500-ml volumetric flask (Point 2.5) and put it in a 250-ml volumetric flask; dilute it with distilled water at the mark, and shake the cylinder. Put 25 ml of the salt solution in the 250-ml beaker in a 150-ml conical flask; add 25 ml of distilled water, 5 – 10 drops of 10% potassium chromate solution (light yellow). Begin the titration by 0.1N silver nitrate solution until the solution turns to a red-brown color.
2.6.4. Result calculation.
The content of Chlorine ions is expressed as percentage of dry matter (X3) according to the following formula:
![]()
0.00355: Amount of chloride ion corresponding to 1 ml of AgNO3 solution (0.1N ), expressed in gram.
V: Volume of AgNO3.(0.1N) that is consumed when titrating, expressed in ml.
200: Dilution factor ![]()
Find the arithmetic mean of 2 parallel calculations. The difference between two parallel calculations is smaller than 0.1%.
2.7. Determination of sulfate ion content (SO4--) (Arbitration method).
2.7.1. Principle: This method is based on the precipitate of SO4-- in the form of barium sulfate by barium chloride, , thence calculate the content of SO4--.
2.7.2. Reagents and tools.
- Hydrochloric acid, 10% solution (pH= 5 – 6).
- 0.1N Barium chloride solution.
- Furnace capable of producing a temperature of 650 ± 5 oC.
2.7.3. Testing process.
Take 100 ml of salty water from the 500-ml volumetric flask to a 200-ml beaker, add 3 ml of HCl 100% and boil them. Heat 10 ml of BaCl2 close to the boiling point, and put the hot BaCl2 solution into the salt solution. Use a glass rod to stir the solution for 3 minute and let it cool down to ambient temperature (no precipitate is seen when some drops of BaCl2 is put into the solution)
Filter out precipitate by thick filter paper. First filter the upper part of precipitate, then add 25 ml of boiled distilled water to the beaker. Repeat this process 5 times then put all precipitate on filter paper, rinse the beaker and wash the precipitate 5 times by hot distilled water until the filtered water does not cause a chlorine reaction with 0.1N AgNo3 .
Take the filter paper that have BaSO4 precipitate out of the funnel and put it on a glass dial. Heat it at 105 oC for 1 hour, then move the precipitate to a capped heated bowl (a dried bowl with known weight) Heat the bowl with the precipitate at 650 ± 50 oC for 1 hour; cool it in a desiccator, then weigh the precipitate. Repeat this process until the weight difference is smaller than 0.001 gram.
2.7.4. Result calculation.
The content of sulfate is expressed as percentage of dry matter (X4) according to the following formula:
![]()
0.445 : Factor of conversion from BaSO4 to SO- -.
m1: Weight of the heated bowl with the precipitate, expressed in gram.
m2: Weight of the heated bowl without precipitate, expressed in gram.
m: Weight of salt, expressed in gram.
V: Volume of the volumetric flask, expressed in ml.
v: Volume of the solution taken from the volumetric flask, expressed in ml.
The result is the arithmetic mean of 2 parallel calculations. The difference between 2 parallel calculations shall not exceed 0.1%.
2.8. Determination of sulfate ion content (SO4--)
2.8.1. Principle.
Use amount of barium chloride intended to form sulfate (SO4--) precipitate of a salt in weak acid (precipitate of BaSO4).
Use Trilon B to titrate, the indicators are eriochrome black T and methyl red.
Use Trilon B to titrate the amount of used BaCl2, Ca++ and Mg++ of salty water, with the same environment and indicators as above.
The content of SO4-- shall be found from the third titration.
2.8.2. Reagents and tools.
- 0.025M Trilon B solution.
- Barium chloride, 0.05M solution.
- Buffer: NH4OH - NH4Cl, pH = 10.
- Indicator: ET00 0.5%
- Methyl red, (0.2% solution).
- Strong hydrochloric acid.
- Burette.
2.8.3. Testing process.
Take 20 ml of salty water from the 500-ml volumetric flask (Point 2.5) to a 250-ml conical flask, add 30 ml distilled water and 1 drop of strong HCl. Add 5 ml of BaCl while shaking the solution. When a white precipitate is seen, allow the solution to stand for 1 – 2 more minutes, then add 2 ml of NH4OH - NH4Cl, 10 drops of ET100, and 10 drops of methyl red. Use Trilon B solution to titrate until the solution turns red, then ashy, then green. Record the amount of used Trilon B (V1).
Take 20 ml of salty water from the 500-ml volumetric flask (Point 2.5) to a 250-ml conical flask, sequentially add 30 ml distilled and 2 ml of Trilon B until the solution color changes as above. Record the amount of Trilon B used (V2).
2.8.4. Result calculation.
The content of SO4-- ions is expressed as percentage of dry matter (X3) according to the following formula:
![]()
96: molecular mass of SO4--
25: Dilution factor ![]()
V1: Volume of Trilon B used for the first titration (titrating Mg+ + , Ca+ + and Ba+ +), expressed in ml.
V2: Volume of Trilon B used for the second titration (titrating 5 ml of BaCl2 solution) expressed in ml.
M: molecular concentration of trilon B.
m: weigh of salt, expressed in gram.
The result is the arithmetic mean of two parallel calculations. The difference between two parallel calculations shall be smaller than 0.5%.
2.9. Determination of Calcium ion content (Ca++)
2.9.1. Principle.
In the salty solution with a pH of 12, the calcein indicator combines with calcium shall give a red color. When Trilon B is present in a solution, it shall create a complex with calcium and expel calcein in a free form. The solution shall then turn bluish yellow. Based on the amount of Trilon B used, the calcium content in the solution shall be determined.
2.9.2. Reagents and tools.
- 0.01N Trilon B solution.
- 0.5% calcein indicator
- 1N sodium hydroxide solution.
- Burette.
2.9.3. Testing process.
Take 20 ml of salt solution from the 500-ml volumetric flask, add 30 ml or distilled water, 5 ml of 1N NaOH, 1 drop of 0.5% Calcein. Begin the single-color titration to identify the necessary Trilon B.
Prepare a similar specimen to the experiment above and add 90% of the amount of Trilon B recorded in the specimen above. Then add 5 ml of NaOH (1N solution) and 4 drops of 0.5% calcein. Use a microburette to keep titrating by Trilon B until the solution turns red and then bluish-yellow. Record the total amount of Trilon B after 2 titrations.
2.9.4. Result calculation
The content of calcium is expressed as percentage of dry matter (X5) according to the following formula:
![]()
0.0004 : Amount of calcium corresponding to 4ml Trilon B, expressed in gram.
V1: Volume of 0.01N Trilon B solution consumed during the titration, expressed in ml.
V2: Volume of salt solution taken from the volumetric flask, expressed in ml.
500: Capacity of the volumetric flask, expressed in ml.
m: Weight of the salt sample, expressed in gram.
The result is the arithmetic mean of two parallel calculations. The difference between two parallel calculations shall be smaller than 0.05%.
2.10. Determination of magnesium ion content (Mg++)
2.10.1. Principle.
This method is based on the titration of total Ca+ +, Mg+ + by Trilon B titrant. The indicator is eriochrome black T in an environment with a pH of 10. The magnesium content shall be determined by combining this process and the method of calcium determination (Point 2.9).
2.10.2. Reagents and tools.
- 0.01N Trilon B solution.
- Eriochromee black T crystals.
- Buffer: NH4OH - NH4Cl, pH = 10.
- Burette.
2.10.3. Testing process.
Take 20 ml of salt solution from the 500-ml volumetric flask and put it in a 250-ml conical flask, then add 30 ml or distilled water, 10 ml of buffer with pH = 10, and some ETOO crystals.
Use 0.01N Trilon B to titrate until the solution completely turns from red to blue. Record the amount of consumed Trilon B.
2.10.4. Result calculation
The magnesium ion content is expressed as percentage of dry matter (X6) according to the following formula:
![]()
0. 00024: Amount of magnesium corresponding to 1 ml of 0.01N Trilon B, expressed in gram.
V2: Volume of 0.01N Trilon B solution that was used to titrate Mg++, expressed in ml.
V2: Volume of 0.01N Trilon B solution that was used to titrate Ca++, expressed in ml (Point 2.9 with the same amount as the determination of Mg++).
V: Volume of salt solution taken from the volumetric flask, expressed in ml.
m: weigh of salt, expressed in gram.
The result is the arithmetic mean of two parallel calculations. The difference between two parallel calculations shall be smaller than 0.5%.
2.11. Determination of potassium ion content (K+)
2.11.1. Principle.
In weak acid, sodium tetraphenylborate reacts with K+ and forms a white precipitate that barely dissolves in water. Heat and filter out the precipitate to determine the potassium content.
2.11.2. Reagents and tools.
- Sodium tetraphenylborate.
Dissolve 3 g of sodium tetraphenylborate NaB(C6H5)4 and 0.5 g of aluminum nitrate Al(NO3)3 in water. Distill 10 ml of the solution and allow it to stand for one night (only mix before use);
- 1N acetic acid.
Washing solution: use 100 ml of distilled water mixed with 2ml of 3% sodium tetraphenylborate and 2ml of 1N acetic acid.
- A filter funnel No. 4
2.11.3. Testing process.
Take 20 ml of salt solution from the 500-ml volumetric flask (Point 2.5) and put it in a 250-ml volumetric flask, then add water until it reaches the mark.
Take 50 ml of solution from the 250-ml volumetric flask, put it in a 150-ml beaker. Acidize it by 5 ml of 1N acetic acid. Boil it at 40 – 50 oC. Slowly add 10 ml of 3% sodium tetraphenylborate. Stir the solution and cool it down to ambient temperature.
Use the funnel No. 4 (heated until its weight remains unchanged at 120 ± 5 oC. Filter out the precipitate. Wash the precipitate 4 times by washing solution right on the funnel, each time 3 ml, and 3 times by distilled water, each time 3 ml. Filter out and dry the precipitate (together with the funnel) at 120 ± 5 oC for 1 hour. Cool it in a desiccator and weigh it.
Repeat the process of heating, cooling and weighing until the weight remains unchanged.
2.11.4. Result calculation
The potassium ion content is expressed as percentage of dry matter (X7) according to the following formula:
![]()
0.1091: Potassium conversion factor from NaB(C6H5)4.
125: Dilution factor ![]()
M: Weight of the precipitate, expressed in gram.
m: Weight of the salt sample, expressed in gram.
The result is the arithmetic mean of two parallel calculations. The difference between two parallel calculations shall be smaller than 0.5%.
2.12. Determination of sodium ion content (Na+)
2.12.1. Principle.
Use potassium antimony K2H2Sb2O7 to form a precipitate if sodium in a neutral environment or weak acid. Dissolve the precipitate in a mixture of hydrochloride acid and potassium iodide, then use sodium thiosulfate to , and thence calculate the Na+ content.
First Mg+ + and Ca+ + ions must be removed from the solution by making it react with oxygen quioline to form a precipitate of Mg(C9H6NO2)2.4H2O.
To reduce the solubility of Na2H2Sb2O7.4H2O in water, the precipitate shall be form in Ethanol solution.
2.12.2. Reagents and tools.
- A solution of 8 2% aniolin oxygen in Ethanol.
- Precipitation solution: dissolve 20g potassium antimony in 1 litre of boiled distilled water. Cool it and add 1g of KOH. Allow it to stand for one night and filter out the sediment.
- 0.05N sodium thiosulfate solution.
- Anhydrous Ethanol.
- Strong hydrochloric acid.
- Potassium iodide crystals.
- Starch, 0.5% ammonium hydroxide solution.
- Heated bath.
2.12.3. Testing process.
Take 100 ml of salty water from the 500-ml volumetric flask (Point 2.5) to a 150-ml beaker. Slightly boil the solution. Then add 10 ml of 2% 8_ Oxy Aninolin solution and 0.3 ml of 10% NH4OH. Boil the solution in the heated bath for 1 -2 minutes and let it cool down; filter out the precipitate on the funnel No. 4. Use 100 ml of distilled water to wash the precipitate many times (in this way Mg++ and Ca++ have been taken out of the solution.
Concentrate the solution above until its volume reaches 25 ml, and add 25 ml of anhydrous ethanol.
Heat this solution in a heated bath and gradually add 25 ml of K2H2Sb2Os while stirring it. Speed up this process when a precipitate is formed.
Allow it to stand at ambient temperature for 1 hour then filter out the precipitate on the funnel No. 4 and wash 7 times by ethanol solution.
Move all precipitate on the funnel to a 150-ml beaker and dissolve the precipitate by 8 ml of strong HCl. Wash the filter funnel and the beaker with distilled water and add more distilled water until the volume reaches about 50 ml. Add 1g of KI crystal and allow it to stand for 5 minutes. Then titrate it against sodium thiosulfate solution until the solution turns light-yellow. Add 5 ml of 0.5% starch indicator. Shake and keep titrating until the solution is colorless.
2.12.4. Result calculation
The sodium ion content is expressed as percentage of dry matter (X9) according to the following formula:
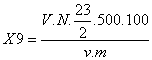
V: Volume of Na2S2O3 consumed during the titration, expressed in ml.
N: Concentration of Na2S2O3.
v: Amount of salt water taken, expresses in ml.
m: Weight of the salt sample, expressed in gram.
![]() : Sodium conversion factor
: Sodium conversion factor
500: Capacity of the volumetric flask, expressed in ml.
The result is the arithmetic mean of two parallel calculations. The difference between two parallel calculations shall be smaller than 0.1%.
2.13. Demonstration of salt composition.
After knowing the contents of ions in salt, the compounds must be demonstrated as follows:
- The combination of Ca++ and SO4-- is expressed as CaSO4.
- Deduct the amount of SO4-- that reacts with Ca++ from the total amount of SO4--, and combine the remainder with MgSO4
- Deduct the amount of Mg+ + that reacts with SO4—from the total amount of Mg++, and combine the remainder with Chlorine to form MgCl.
Summary of salt composition demonstration.
|
Cation Anion |
Ca+ + |
Mg+ + |
K+ |
Na+ |
|
SO4- - Cl- |
CaSO4 |
MgSO4 MgCl2 |
KCl |
NaCl |
Note: The sodium content determined by the method in Point 2.2 may be combined with Chlorine to demonstrate the composition of NaCl. If the result does not matches the table above, that figure may be used for reference.
