Nội dung toàn văn Official Dispatch 1734/BYT-DP 2021 guidelines for organization of COVID19 vaccination sessions
|
MINISTRY OF
HEALTH |
SOCIALIST
REPUBLIC OF VIETNAM |
|
No. 1734/BYT-DP |
Hanoi, March 17, 2021 |
To: Departments of Health
On 05/3/2021, the Ministry of Health promulgated Decision No. 1467/QD-BYT approving 2021-2022 COVID-19 vaccination plan and Decision No. 1464/QD-BYT on guidelines on receiving, preserving, distributing and using COVID-19 vaccine.
In order to organize safe and efficient COVID-19 vaccination sessions, the Ministry of Health formulates the guidelines for organization of COVID-19 vaccination sessions; and requests Departments of Health to provide direction and guidance on, inspect and supervise proper organization of COVID-19 vaccination sessions by vaccination facilities based on these guidelines.
During the implementation of these guidelines, any difficulty arising should be reported to General Department of Preventive Medicine - Ministry of Health (phone number: 0243.8462364, email: [email protected]).
|
|
P.P. THE MINISTER |
GUIDELINES
ORGANIZATION OF COVID-19 VACCINATION SESSIONS
(Promulgated together with Official Dispatch No. 1734/BYT-DP dated 17/3/2021 by Ministry of Health)
For safe and efficient vaccination operations, the Ministry of Health provides the guidelines for organization of COVID-19 vaccination sessions with the following contents:
I. GENERAL INFORMATION
1. Available COVID-19 vaccines:
- Inactivated vaccines.
- Viral vector vaccines.
- Recombinant protein vaccines.
- DNA vaccines.
- RNA vaccines.
- Capsid vaccines.
- Attenuated vaccines.
As of 22/02/2021, WHO has approved the following 03 COVID-19 vaccines for emergency use: Pfizer-BioNTech COVID-19 mRNA vaccine, AstraZeneca/SKBio vaccine and Serum Institute of India Pvt Ltd - COVISHIELD™. The Ministry of Health has promulgated Decision No. 983/QD-BYT dated 01/02/2021 on conditional approval for COVID-19 vaccines for emergency use.
2. Dosage: most available COVID-19 vaccines require 2 doses (interval between 2 doses is given by the manufacturer).
3. Injection route: mostly intramuscular injection.
4. Storage conditions: most COVID-19 vaccines must be stored at 2-8 degrees Celsius; some vaccines require ultra-cold storage.
5. Expiry date: between 6 - 9 months according to each manufacturer’s recommendation.
II. CONTENT
1. Vaccination facilities
Vaccination facilities must be eligible to administer COVID-19 vaccines and shall administer COVID-19 vaccines in compliance with Decision No. 1464/QD-BYT dated March 05, 2021 by the Ministry of Health on guidelines on receiving, preserving, distributing and using COVID-19 vaccine.
2. Pre-vaccination preparation
2.1. Investigation, formulation of vaccine recipient lists and formulation of vaccination plans
- Based on the Ministry of Health’s plans, Departments of Health shall take charge and cooperate with regulatory bodies and People’s Committees of districts in their provinces in formulating and proposing plans for local COVID-19 vaccine use to the People’s Committees of their provinces for approval; and directing development of vaccine recipient lists for each vaccination round.
- Information on vaccine recipients to be consolidated includes: full name, date of birth, address, ID number, phone number, occupation and health insurance card number (consolidated by recipient group according to the Government’s Resolution No. 21/NQ-CP dated 26/2/2021) (using the form in Appendix 1).
- Vaccination facilities shall formulate plans for their vaccination sessions: draw up list of vaccine recipients by time slot, ensuring that there are no more than 100 recipients per one vaccination point per one vaccination session; assign and announce the vaccination time of each recipient group or each village, hamlet or unit receiving the vaccine.
2.2. Facilities
- Provide spacious areas for pre-vaccination waiting, screening, consultation, vaccine administration, 30-minute monitoring and handling of adverse events following immunization with sufficient seating and safe distance between vaccine recipients, families thereof and healthcare workers.
- Arrange the vaccination process in one direction, and ensure safe distance between vaccination tables/ locations for disease prevention purpose, which shall be placed in the following order: welcome and instruction table → pre-vaccination waiting area → pre-vaccination screening and consultation table → vaccination table → vaccination documentation table → area for monitoring and handling of adverse events following immunization.
- Provide and clean restrooms using a disinfectant solution on a daily basis.
2.3. Equipment
- Prepare sufficient equipment, tools and materials used for vaccination and vaccination forms.
- Prepare equipment for immediate emergency aid and detailed plans for emergency aid where necessary.
- Provide wash basins and soap or liquid hand wash for vaccination points.
- Frequently-touched surfaces must be cleaned using suitable measures (at least once every session when there is community transmission; otherwise, at least once a day).
- Provide hand sanitizer and face masks at the entrance and locations frequently touched by vaccine recipients and healthcare workers (doorknobs, elevators, etc.).
- Place tools on the vaccination table in an order convenient for healthcare workers. Each vaccination table shall have equipment necessary for vaccine storage and administration such as vaccine carriers, syringes, clamp holders, clamps, dry cotton ball and alcohol cotton ball holders, Adverse Events Following Immunization (AEFI) kit and pens. Do not place medications or sample containers on vaccination tables. Safety boxes, vaccine vial containers and garbage bins shall be placed at appropriate locations.
- Required professional documents; posters and leaflets providing instructions on vaccination steps and schedules for vaccination, monitoring, care and handling of adverse events following immunization shall be posted on the wall at vaccination locations for healthcare workers, vaccine recipients and people to read.
2.4. Workforce
- Workforce shall be provided in accordance with regulations in the Government’s Decree No. 104/2016/ND-CP dated 01/06/2016 on vaccination and Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health.
- Vaccination workforce must receive training in COVID-19 safety and monitor their health conditions according to guidelines of the Ministry of Health.
- Each vaccination point must have a detailed task assignment table.
2.5. Maintaining hygiene for COVID-19 prevention
- Vaccination workforce and vaccine recipients and families thereof must take personal protective measures such as use of face masks and regular hand washing or hand disinfection.
- Avoid talking or having contact with other people at vaccination points.
3. Vaccination steps
COVID-19 vaccination steps
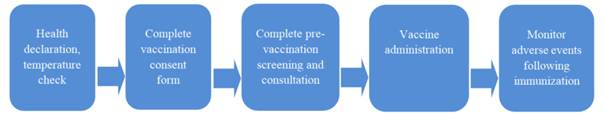
Step 1: Receive and classify vaccine recipients at the welcoming area; provide instructions on electronic health declaration and check that vaccine recipients and their families have declared their health conditions; provide face masks for vaccine recipients (if they are not wearing one); check temperature of vaccine recipients.
Step 2: Provide the COVID-19 vaccination consent form (which is made using the form in Appendix 2 enclosed therewith) for vaccine recipients to read, fill out and sign if they agree to receive the vaccine.
Step 3: Screen vaccine recipients before vaccination according to guidelines of the Ministry of Health. Explain vaccine effects and benefits and possible post-immunization reactions to vaccine recipients and their guardians. Inform vaccine recipients and their guardians of the effects, dosage and administration route of the vaccine being used. Provide information on monitoring after COVID-19 vaccination.
Step 4: Administer the vaccine according to indications and ensure safety in compliance with regulations in Article 11 of Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health.
Note:
- Vaccination facilities in hospitals receiving COVID-19 patients may request the vaccination workforce to wear protective equipment if necessary based on the situation and risk assessment.
- Use vaccine carriers to store vaccines at vaccination tables.
4. After vaccination
- Monitor vaccine recipients for at least 30 minutes after the vaccine is administered and provide vaccine recipients or their families with instructions on how to monitor their general condition, mental condition, eating, sleeping, breathing, urticarial and symptoms at the injection site for 24 hours and 7 days after vaccination and notify healthcare workers immediately upon any abnormal sign. Vaccine recipients shall contact a hospital or healthcare facility if developing high fever (≥39°C), cyanosis, breathing difficulty, etc. after vaccination or when common reactions last for more than 24 hours after vaccination.
- Healthcare facilities receiving vaccine injury cases must provide emergency aid and treatment for these cases and report to the Department of Health within 24 hours after receiving these persons. Consolidated reports on vaccine injury cases shall be prepared and submitted according to regulations in Article 6 of the Government’s Decree No. 104/2016/ND-CP dated 01/7/2016 and Articles 14, 15 and 16 of Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health.
- Waste shall be collected, stored, transported and treated according to regulations in Joint Circular No. 58/2015/TTLT-BYT-BTNM dated 31/12/2015 by the Ministry of Health and Ministry of Natural Resources and Environment stipulating regulations on biomedical waste management, Official Dispatch No. 102/MT-YT dated 04/03/2021 by Health Environment Management Agency on guidelines for management of biomedical waste in COVID-19 vaccination and relevant documents.
5. Recording and reporting
- Record all necessary information in vaccination sheets or books of vaccine recipients and on the vaccination information management software and notify vaccine recipients of the schedule for the next dose; input the vaccination date and adverse events following immunization into the vaccination information management software. Provide persons having received all doses with a confirmation of vaccination, which is made using the form in Appendix 3 enclosed therewith.
- Requirements, formats, submission procedures, deadlines and contents of periodic and ad hoc reports are provided for in Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health.
- Requirements, formats, submission procedures and deadlines of daily reports are provided for in Circular No. 34/2018/TT-BYT dated 12/11/2018 by the Ministry of Health. These reports shall be formulated using the form in Appendix 4 enclosed therewith.
APPENDIX 1
LIST OF COVID-19 VACCINE RECIPIENTS (Excel file)
Province/City……………………….District...........................
Commune……………………………..Vaccination point.........
|
No. |
Full name* |
Date of birth |
Age |
Sex |
Prioritized recipient group code (from 1 to 10) |
Workplace |
Phone number* |
ID card number* |
Health insurance card number* |
Current address* |
Date of 1st dose |
Date of 2nd dose |
NOTE |
|||
|
House no./Neighborhood |
Commune/Ward |
District |
Province/City |
|||||||||||||
|
1 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
2 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
3 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
4 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
5 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
6 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
7 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
8 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
9 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
|
10 |
|
…/../.... |
|
|
|
|
|
|
|
|
|
|
|
…/…/20… |
…/…/20… |
|
*: REQUIRED FIELD
Prioritized recipient group codes: (1) Frontline workers; (2) Vietnamese diplomatic staff and officials working abroad; customs staff and officials involved in entry and exit operations; (3) Providers of essential services such as aviation, transport, tourism, power and water supply, etc.; (4) Teachers and those working in educational and training institutions; those working for administrative units and bodies and frequently having contact with multiple people; (5) Those with chronic diseases, those aged above 65; (6) Residents in pandemic-hit areas; (7) Poor people, policy beneficiaries; (8) Those who will be sent abroad for business, learning or working by competent regulatory agencies; (9) Other people subject to decision of the Ministry of Health depending on COVID-19 situation; (10) Other, specify………..
|
List maker |
[Location and date] Vaccination unit (signature and stamp) |
APPENDIX 2
|
COVID-19 VACCINATION CONSENT FORM 1. Vaccination is an effective preventive measure; however, COVID-19 vaccines might not offer full protection against the disease. People who are fully vaccinated against COVID-19 may be protected from the disease or have less severe illness. People who have received a COVID-19 vaccine should still follow the 5K requirements for COVID-19 prevention. 2. COVID-19 vaccination may result in some manifestations of localized injection-site or systemic reactions such as swelling or aching at injection site, headache, nausea, fever, muscle ache, etc. or vaccine injury. 3. Upon detection of any abnormal symptom, vaccine recipients should contact the nearest healthcare facility for timely advice, examination and treatment. After reading the above information, I understand the risks and: agree to receive the vaccine □ do not agree to receive the vaccine □ Full name of vaccine recipient:…………………………………………….. Phone number:……………………………..
|
APPENDIX 3
CONFIRMATION OF COVID-19 VACCINATION Full name:........................................................................................................ Date of birth: .................................................................................................. Phone number:................................................................................................. Address:.......................................................................................................... Has received the following COVID-19 vaccine doses:
|
APPENDIX 4
Form No. 1
DAILY REPORT ON IMPLEMENTATION OF COVID-19 VACCINATION PLAN
(For vaccination facilities)
Province/City………………………District…………………………………
Vaccination facility…………………….
Total number of people registering for vaccination: ……………..,
Date ……………
|
Number of vaccine recipients |
Amount of vaccine received |
Amount of vaccine used |
Amount of vaccine disposed of |
Amount of vaccine remaining |
Number of postponed vaccinations1 |
Number of cases with common reactions2 |
Number of vaccine injury cases3 |
|
|
|
|
|
|
|
|
|
Note: if the facility administers the vaccine to multiple units, use the following table:
|
Name of unit |
Number of vaccine recipients |
Amount of vaccine received |
Amount of vaccine used |
Amount of vaccine disposed of |
Amount of vaccine remaining |
Number of postponed vaccinations1 |
Number of cases with common reactions2 |
Number of vaccine injury cases3 |
|
Unit 1 |
|
|
|
|
|
|
|
|
|
Unit 2 |
|
|
|
|
|
|
|
|
1 Report on postponed vaccinations
Number of postponed vaccinations: ____________
Other reasons:………………………..
2 Detailed report on cases with common reactions
|
Common reactions |
|||
|
Unit |
Fever ≤ 39°C |
Swelling/Aching at injection site |
Other symptoms |
|
Unit 1 |
|
|
|
|
Unit 2 |
|
|
|
3 Report on vaccine injury cases:
Brief description of each case:
|
No. |
Unit |
Full name |
Date of birth |
Description (symptoms, developments, treatment, result, etc.) |
|
|
|
|
|
|
|
|
|
|
|
|
Vaccine injury shall be reported on a case-by-case basis using the vaccine injury investigation sheet in Circular No. 34/2018/TT-BYT dated November 12, 2018.
|
Reporter (signature and
full name) |
… / … / 2021 Head of facility (signature and
stamp) |
APPENDIX 4
Form No. 2
CONSOLIDATED DAILY REPORT ON IMPLEMENTATION OF COVID-19 VACCINATION PLAN
(For provinces/central-affiliated cities)
Province/City...............................
Total number of people registering for vaccination: ……………..,
Date ……………
|
Name of healthcare facility |
Number of vaccine recipients |
Amount of vaccine received |
Amount of vaccine used |
Amount of vaccine disposed of |
Amount of vaccine remaining |
Number of postponed vaccinations1 |
Number of cases with common reactions2 |
Number of vaccine injury cases3 |
|
Facility 1 |
|
|
|
|
|
|
|
|
|
Facility 2 |
|
|
|
|
|
|
|
|
1 Report on contraindication cases
Number of postponed vaccinations: ____________
Other reasons:…………………………………………..
2 Detailed report on cases with common reactions
|
Common reactions |
|||
|
Unit |
Fever ≤ 39°C |
Swelling/Aching at injection site |
Other symptoms |
|
Unit 1 |
|
|
|
|
Unit 2 |
|
|
|
3 Report on vaccine injury cases:
Brief description of each case:
|
No. |
Unit |
Full name |
Date of birth |
Description (symptoms, developments, treatment, result, etc.) |
|
|
|
|
|
|
|
|
|
|
|
|
Vaccine injury shall be reported on a case-by-case basis using the vaccine injury investigation sheet in Circular No. 34/2018/TT-BYT dated November 12, 2018.
|
Reporter (signature and
full name) |
… / … / 2021 Head of facility (signature and
stamp) |