Nội dung toàn văn Official Dispatch 8095/SYT-NVY 2021 control the COVID19 epidemic in manufacturing facilities
|
PEOPLE’S
COMMITTEE OF HO CHI MINH CITY |
SOCIALIST
REPUBLIC OF VIETNAM |
|
No. 8095/SYT-NVY |
Ho Chi Minh City, November 1, 2021 |
|
To: |
- Ministries and Departments in the
City; |
Pursuant to:
- Resolution No. 128/NQ-CP dated October 11, 2021 of the Government on Provisional Guidelines on “Safety, flexibility, and effective control of COVID-19”;
- Resolution No. 4800/QD-BYT dated October 12, 2021 of Ministry of Health on medical specialty for implementation of Resolution No. 128/NQ-CP dated October 11, 2021 of the Government,
- Official Dispatch No. 3498/UBND-VX dated October 21, 2021 of People’s Committee of the City regarding implementation of procedures and guidelines relating to epidemic management in manufacturing facilities and business entities,
In order to facilitate operation of manufacturing facilities, business entities, industrial parks, socio-economic development, and implementation of COVID-19 epidemic management in the City, Department of Health implements Epidemic management plans in manufacturing facilities, business entities, and industrial parks attached to the following Annexes:
1. Annex 1: Procedures for handling persons infected with COVID-19 in manufacturing facilities and business entities
2. Annex 2: Local medical teams in COVID-19 epidemic management
3. Annex 3: Commitment for implementation of COVID-19 epidemic management with supervisory entities.
For your implementation. Difficulties that arise during implementation hereof should be reported to Department of Health for cooperation and resolution./.
|
|
PP. DIRECTOR |
PLAN
FOR COVID-19 EPIDEMIC MANAGEMENT
IN MANUFACTURING FACILITIES, BUSINESS ENTITIES, AND INDUSTRIAL PARKS
(Attached to Official Dispatch No. 8095/SYT-NVY dated November 1, 2021 of Department of
Health)
I. OBJECTIVES
1. Actively prevent and control the epidemic in enterprises and manufacturing facilities following the principles of controlling infection risk, detecting infected individuals prematurely, quarantining, localizing, and preventing outbreak within the entities.
2. Implement epidemic management and guarantee safe working environment for workers while guaranteeing operation of enterprises.
II. REQUIREMENTS
1. Pursuant to guidelines and general provisions of the Steering Committee for COVID-19 Epidemic Management of the City regarding infection level-based COVID-19 epidemic management plan in specific field of operation, enterprises shall actively develop operational plan depending on epidemic level, financial situation, and operational characteristics of the enterprises.
2. The plan must meet epidemic management requirements, and guarantee order and security, fire safety, food safety, and environmental hygiene, etc.
III. COVID-19 EPIDEMIC SAFETY MEASURES
Manufacturing facilities, business entities, and industrial parks shall assign their medical teams (according to Annex 2) or enter into contracts with (public/private) medical entities in order to implement and supervise compliance with epidemic safety measures during operations
1. For workers: guaranteeing any of the following
- Workers have received sufficient doses of COVID-19 vaccine.
- Workers have received at least 1 dose of COVID-19 vaccine and are included in vaccination plan for the second dose.
- Workers who were infected with SARS-CoV-2, have relieved of SARS-CoV-2, and have finished quarantine, medical supervision as per the law.
2. For movement
- Worker transport vehicle:
+ Transport vehicles must be sterilized after each trip
+ Vehicle operator and assistant must receive adequate vaccine does and must keep daily record of physical contact
+ Examine body temperature and request workers to wash hands prior to boarding the vehicle
+ Number of passengers on the vehicle must conform to specific requirements for epidemic management from time to time. If all workers have received 2 doses of COVID-19 vaccine, may carry passengers as per vehicle capacity.
- Workers who travel by themselves: strictly comply with 5K principle as per the law.
3. For workplaces
3.1. Control external sources of infection
- Organize medical declaration (mandatory for workers, partners, and contractors providing services and commodities for enterprises) prior to entering the entities.
- Measure body temperature prior to entering working position.
- For individuals reaching for professional matters: specify range of movement within the workplace
- Upon detecting individuals with signs of COVID-19 infection or epidemiology factors, screening department must immediately inform medical teams or report to head of the entities or enterprises and contact medical unit to conduct SARS-CoV-2 test as per the law.
3.2. Control sources of infection at workplace
- Organize airy workplace and assign equipment in a manner that guarantees ventilation for the workplace.
- Workers must comply with 5K and maintain a minimum distance of 1 m throughout working process.
- Prepare sufficient soap/hand sanitizer in convenient areas that allow workers to wash their hands regularly.
- Assign workers to have meal in shifts; request workers to take designated seats when having meal, maintain 2 m of distance, not sit face-to-face, install partitions, and separate routes leading to and from the cafeteria
- Sanitize and sterilize on a periodic basis:
+ In public area: 1 time/day
+ In manufacturing area: after each shift
+ Restroom area: 3 – 4 times/day, after break time of workers
- Equip surveillance camera and supervise compliance with epidemic management regulations in public area and manufacturing area.
- Prepare operational procedures in a manner that reduces physical contact between departments of enterprises.
- Supervise and detect suspected cases via medical declaration, investigate reasons for taking leave of workers, and monitor health of workers throughout working period.
- Organize random SARS-CoV-2 test for individuals facing high risk of COVID-19 infection according to specific guidelines of the medical sector.
- Upon detecting individuals tested positive to SARS-CoV-2 at workplaces or enterprises, medical team (or medical entities that have entered into contract with the workplaces or enterprises) shall implement F0 handling procedures according to guidelines of medical sector (Annex 1).
4. For accommodation of workers:
- Enterprises shall cooperate with local authority in designating and enabling workers to have safe accommodation
- Recommend arranging individuals working in the same position in the same area in order to restrict infection.
- Accommodation must be clean and well-ventilated.
IV. ORGANIZATION FOR IMPLEMENTATION
1. Management Board of Export-processing zones and Industrial parks, Management Board of Hi-tech zones, Quang Trung Software Company (industrial park)
- Direct the entities to develop infrastructure with adequate conditions and cooperate with competent authority in establishing F0 healthcare areas in industrial parks
- Cooperate with medical sector and local government in organizing vaccination for workers who have not received sufficient doses of COVID-19 vaccine.
- Supervise and assist enterprise in epidemic management as per the law.
2. Steering Committees for COVID-19 epidemic management of Thu Duc City and districts
- Cooperate in assisting enterprises in organizing safe accommodations for workers within their competence.
- Organize COVID-19 epidemic management in the area and guarantee safe manufacturing conditions.
- Organize inspection and supervision of compliance with regulations on epidemic management of the enterprises; monitor epidemic situations in manufacturing facilities, business entities, and industrial parks in the area to serve timely intervention.
- Direct medical centers of districts and Thu Duc City to instruct local manufacturing facilities and business entities to implement COVID-19 safety measures and Handling procedures upon detecting individuals infected with COVID-19 in entities and enterprises; assist in handling F0 and F1 in enterprises as per the law.
3. Manufacturing facilities, business entities, and industrial parks
- Establish department assuming epidemic management namely the medical team of the entities or enter into contract with medical facilities to be consulted regarding epidemic management measures, provided with testing and environmental hygiene services, etc. at request of the entities.
- Develop COVID-19 epidemic management plan in enterprises according to applicable regulations and guidelines, and implement measures for preventing infection.
- Designate temporary quarantine areas satisfactory to regulations of Minsitry of Health. If an enterprise enters into contract with a medical facility or an industrial park develops F0 healthcare area, the temporary quarantine area is not required to be equipped with hospital beds but areas where infected individuals wait for transfer to quarantine area.
- On the basis of realistic conditions of enterprises, develop specific solutions for dealing with cases where COVID-19 infected individuals are found in the enterprises according to procedures issued by Department of Health; inform every enterprise in the area for adoption.
- Implement COVID-19 testing in enterprises:
+ Develop plan for organizing random SARS-CoV-2 test for for individuals facing high risk of COVID-19 infection according to specialized guidelines of medical sector
+ Inform Management boards (for enterprises in Export-processing zones, industrial parks, Hi-tech zones, and Quang Trung Software City), medical centers of districts, Thu Duc City, and Center for Disease Control (CDC) of the City about testing plan for supervision.
+ Organize testing for workers according to the plan: testing method can be RT-PCR (individual sampling or group sampling) or quick test (individual sampling or group sampling with biological permitted by Ministry of Health); may enter into contract with medical entities for provision of RT-PCR test or enable medical personnel of enterprises, hired medical personnel to conduct quick test or enable workers of the enterprises to test after being instructed;
+ In case an individual is tested positive for COVID-19, the enterprise must immediately inform local medical center and CDC of the City and comply with issued procedures
+ Enterprises shall submit testing report to Management Board of industrial parks, CDC of the City, medical centers of Thu Duc City and districts (within 2 hours after concluding the testing day);
+ Assume legal responsibilities for quality, procedures, and results of the antigen test.
4. Workers
- Perform medical declaration on the basis of adequacy and truthfulness
- Vaccinate adequately as per schedule
- Comply with regulations on epidemic management at workplaces and accommodations
5. CDC of the City
- Assist, supervise development and implementation of Plan for COVID-19 epidemic management of enterprises (within their competence), and provide specialized assistance for medical centers when needed.
- Instruct and direct medical centers of districts and Thu Duc City to conduct random, periodic screening tests in manufacturing facilities, business entities, and industrial parks with high infection risk in order to supervise infection risk on a regular basis and prematurely detect potential epidemic cluster in order to control and prevent outbreak.
ANNEX 1
HANDLING PROCEDURES UPON
FINDING INDIVIDUALS INFECTED WITH COVID-19 IN MANUFACTURING FACILITIES AND
BUSINESS ENTITIES
(Attached to Official Dispatch No. 8095/SYT-NVY dated November 1, 2021 of Department of
Health)
Individuals having positive RT-PCT or quick antigen test results are considered to be persons infected with COVID-19 (collectively referred to as “F0”).
Manufacturing facilities and business entities (hereinafter referred to as “units”) must establish medical teams which act as specialized departments and are tasked with monitoring health of workers, supervising compliance with regulations and law on epidemic management, and implementing handling procedures upon finding individuals tested positive for SARS-CoV-2. Medical teams must consist of full-time medical personnel of the units or medical personnel employed under contracts signed between the units and public/private medical facilities.
All units must supervise to detect F0 prematurely by:
- Monitoring health of workers (via medical declaration, body temperature reading, health monitor during working process) on a daily basis, conducting quick antigen test for workers who display symptoms or are recorded for relevant epidemiology factors.
- Organizing random SARS-CoV-2 test for individuals facing high risk of COVID-19 infection according to specific guidelines of the medical sector in each phase of the epidemic
Note: Do not test individuals who have received sufficient doses of vaccine and individuals who have relieved of COVID-19; only test under epidemiology investigation or when workers arrive from areas under level 4 epidemic or regional medical quarantine.
Handling procedures upon finding persons infected with COVID-19 (F0) in manufacturing facilities and business entities:
Step 1:
- Temporarily isolate F0 in quarantine chambers or quarantine areas of the units and immediately contact local medical authority for assistance:
+ In case of industrial parks and industrial complexes: contact mobile medical stations situated in industrial parks and industrial complexes (if any) or medical centers of districts where the units are operating.
+ In case of other manufacturing facilities and business entities: contact medical stations of communes, wards.
- Assign:
+ Medical teams of the units to temporarily isolate F0
+ Medical stations and medical centers to report to CDC of the City upon receiving information on F0 located in the units
Step 2:
- Assess health condition of F0:
If F0 is found to be suffering from respiratory failure (rapid breathing or dyspnea or SpO2 below 96%): allow oxygen therapy, contact, and transfer F0 to hospitals that have COVID-19 department/ward in the same area, or transfer to the nearest COVID-19 field hostpitals via ambulance. In case a unit has separate treatment facility, use a single dose of anticoagulant according to treatment regimen of Minsitry of Health prior to transferring the patient.
If F0 displays no or mild symptoms: Advice and instruct F0 to assess satisfaction of criteria for quarantining at home (according to Decision No. 4038/QD-BYT dated August 21, 2021 on “Provisional guidelines for managing COVID-19 patients at home”)
+ If persons infected with COVID-19 are eligible for quarantining at home and can prevent infection to other household members, especially individuals with high risk (individuals above the age of 65, individuals above the age of 50 and having underlying medical conditions, overweight individuals with BMI > 5, pregnant women, and women during the 2 weeks postpartum), medical teams shall contact medical centers of districts where the units are operating for management and assistance in transferring information of the F0 to medical centers of districts where the F0 are residing for treatment, provision of medicinal package (A-B and C package) and management during period of quarantining at home as per the law.
+ If persons infected with COVID-19 are ineligible for quarantining at home: F0 shall propose appropriate quarantine location (quarantine facilities of enterprises, concentrated quarantine facilities in communes, districts, etc.)
- Assign medical teams of units to cooperate with mobile medical stations in industrial parks, industrial complexes (if any) or medical stations of communes, wards where the units are operating
Step 3:
- Input F0 information onto “System for managing quarantined individuals and COVID-19 patients”.
+ In case of untis that have concentrated quarantine facilities: input F0 information on “System for managing quarantined individuals and COVID-19 patients” (using the account issued by Department of Health).
+ In case of units that do not have concentrated quarantine facilities: submit reports on lists of F) to medical centers of districts where the units are operating in order to input data onto “System for managing quarantined individuals and COVID-19 patients”.
- Assign for implementation: medical teams of the units
Step 4:
- Cease operation of the area where F0 is located in order to sterilize and test all F1 (depending on the scale of infection cluster), and investigate t o determine scale and nature of infection cluster:
+ F0 is located in 1 line of manufacture: handle on the scale of line of manufacture
+ F0 is located in 2 lines of manufacture or more in the same workshop: handle on the scale of the workshop
+ F0 is located in 2 lines of manufacture or more in different workshops without any epidemiological relationship: handle on the scale of each line of manufacture
+ F0 is located in 2 lines of manufacture or more in different workshops with epidemiological relationship: handle on the scale of the entire manufacturing facility
- Identify F1 (individuals who have made close contact with F0 in under 2 m for longer than 15 minutes such as working next to F0 in line of manufacture, sitting in the same table, etc.) and conduct quick antigen test (group sampling must not consist of more than 3 individuals)
- Monitor F1:
+ In case at least 80% of workers of a manufacturing facility has been adequately vaccinated: all F1 are allowed to work and must be tested on the 3rd and 7th day and every 7th day fterwards until no more F0 is found; adopt measures for preventing infection for household members, especially individuals with high risk (individuals above the age of 65, individuals above the age of 50 and having underlying medical conditions, overweight individuals with BMI > 5, pregnant women, and women during the 2 weeks postpartum); minimize external contact until supervision period expires.
+ In case less than 80% of workers of a manufacturing facility is adequately vaccinated:
• For F1 who have not been adequately vaccinated: quarantine at home (if eligible) or in quarantine facilities for 14 days; and test again on the 14th day.
• For F1 who have been adequately vaccinated: act similar to the case where at least 80% of workers of the manufacturing facility has been adequately vaccinated
- Assign:
+ Medical teams and Steering Committee for Epidemic Management of the units to organize handling
+ Medical stations and medical centers to assist the units in implementation; if necessary, request assistance of hospitals (in conducting tests, etc.), CDC of the City (in managing complicated infection cluster)
ANNEX 2
MEDICAL TEAM OF THE UNIT IN
COVID-19 EPIDEMIC MANAGEMENT
(Attached to Official Dispatch No. 8095/SYT-NVY dated November 1, 2021 of Department of
Health)
I. POSITION OF MEDICAL TEAM IN EPIDEMIC MANAGEMENT FORCE IN THE UNIT
|
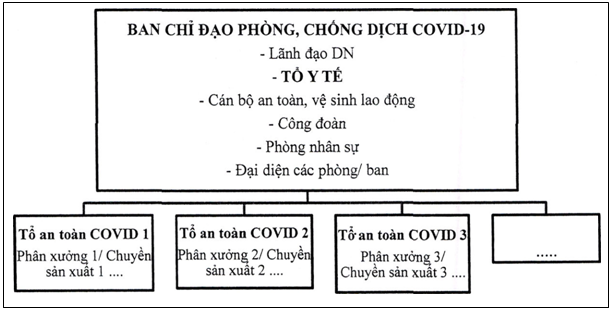 - Steering Committee for COVID-19 epidemic management in
enterprises according to Decision No. 2194/QD-BCDQG dated May 27, 2020:
- Steering Committee for COVID-19 epidemic management in
enterprises according to Decision No. 2194/QD-BCDQG dated May 27, 2020:
|
|
|
||||||
II. TASK OF MEDICAL TEAM OF THE UNIT
1. Managing the epidemic in enterprise
- Contact CDC of the City of medical authority according to regulations of local governments in order to receive guidelines, cooperate in developing plans, and assist implementation of epidemic management.
- Advise heads of enterprise to issue Plan for COVID-19 epidemic management, assign, and publicize contact information (name and phone number) of official(s) in charge of COVID-19 epidemic management at workplace.
- Advise head of enterprise to inform and instruct suppliers of manufacturing materials, services (food, meals, environmental hygiene, sterilization, etc.) to sign commitment for compliance with regulations on COVID-19 epidemic management and supervision regime.
- Organize training and publicize personal hygiene measures and epidemic management measures for workers and customers (if any).
- Propose arrangement and addition of equipment, masks, soap, hand sanitizer, surface disinfectant, and medical material, equipment, etc. for medical departments in the facilities; assign separate area and classify separate care areas for cases where workers show symptoms of fatigue, fever, coughing, sore throat,dyspnea.
- Cooperate with unions/organization representing workers, heads, chiefs of departments, workshops in monitoring health of workers, detecting, and taking timely actions in case workers exhibit symptoms of fatigue, fever, coughing, sore throat, dyspnea. Produce list and monitor health of individuals who make contact with persons who exhibits any symptom such as fever, coughing, dyspnea.
- Upon finding workers who show any symptom such as fatigue, fever, coughing, sore throat, dyspnea at workplace or workers who are infected or individuals who make close contact, handle as per the law. For workplaces that include medical rooms or medical stations, review, arrange, and issue announcement on separate walkway to enable workers who exhibit any symptom such as fatigue, fever, coughing, sore throat, dyspnea to receive examination and advice.
- Request heads of the enterprise to assign/establish teams for inspecting, supervising implementation of epidemic management of workers and departments at workplaces.
- Regularly update COVID-19 epidemic situation, consolidate reports on epidemic management at workplace on a daily, weekly, and monthly basis for workers/Steering Committee for Epdiemic Management of the workplaces.
- Organize periodic screening test as per the law for workers
- Classify, quarantine, and taking care of F0 and F1
- Organize sterilization
2. Implementation of occupational safety and hygiene
- Develop measures and means for providing first aid, emergency response, essential medicines, and occupational accident scenarios, organize training for first aid and emergency response for workers working in the units;
- Develop plans and organize health check-up, occupational disease check-up, medical assessment for identifying reduction of working capacity after suffering from occupational accident and/or occupational disease, healthcare, working capacity recovery, advice for occupational disease preventive measures; propose and arrange working positions suitable for workers’ health;
- Organize regular medical examination and treatment at the unis and first aid, emergency response in case of occupational accidents, technical incidents causing loss of safety, occupational hygiene as per the law;
- Publicize information on occupational hygiene, prevention of occupational diseases, improving health at workplace; inspect compliance with regulations on hygiene, epidemic management organization, food safety and hygiene assurance for workers at workplaces; organize compensation in form of goods as per the law;
- Establish and manage information on hygiene and work at workplaces; organize monitor of working environment in order to assess detrimental factors; manage health profile of workers, health profile of individuals suffering from occupational diseases (if any);
- Cooperate with occupational safety and hygiene department in implementing relevant tasks specified under Clause 2 Article 72 of Law on Occupational Safety and Hygiene.
III. NUMBER OF MEDICAL PERSONNEL BASED ON THE SCALE OF WORKFORCE
|
|
Number of workers (person) |
Medical personnel |
|
1 |
<300 |
1 personnel with level 4 of Vietnamese qualification framework (VQF) |
|
2 |
300 - 500 |
1 doctor/physician and 1 personnel with level 4 of VQF |
|
3 |
500- 1.000 |
1 doctor and 1 personnel with level 4 of VQF |
|
4 |
> 1.000 |
1 doctor and 2 medical employees |
Enterprises that cannot organize medical teams may enter into contracts with public/private medical entities to perform epidemic management and provide healthcare for workers.